- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Publication
Article
Dermatology Times
Risankizumab Efficacious in Clearing, Improving Plaque Psoriasis
Author(s):
AbbVie announced study results demonstrating efficacy in patients with a history of previous interleukin-17 inhibitor therapy.
Risankizumab (Skyrizi) proved safe and efficacious in patients with plaque psoriasis and a history of biologic use, according to a press release1 and late-breaking research session at the American Academy of Dermatology Annual Meeting in New Orleans, LA.
fusssergi/AdobeStock
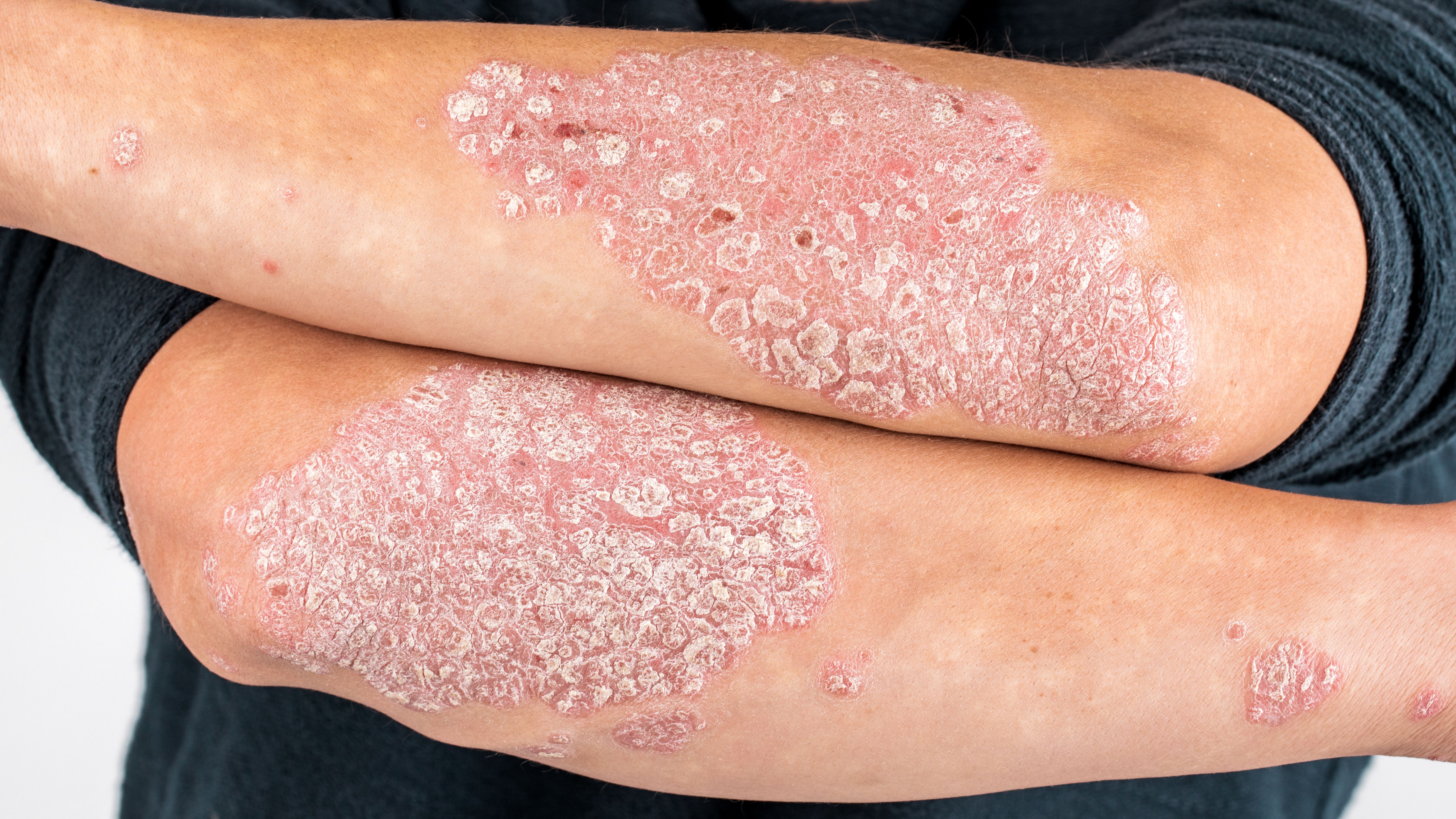
AbbVie, the pharmaceutical biotech company behind the latest results, said risankizumab improved psoriasis signs and symptoms in a recent study.
The study, a 52-week, phase 3, open-label, single-arm study sought to determine the efficacy of risankizumab in patients with moderate-to-severe plaque psoriasis who had previously used interleukin-(IL) 17A treatments.
Conducted at multiple research locations, the study included 252 participants ages 18 and older with moderate-to-severe plaque psoriasis. Participants had also experienced prior suboptimal responses to specific IL-17As such as secukinumab and ixekizumab after at least 6 months of attempted treatment, as defined by a static Physician’s Global Assessment score (sPGA) of 2 or 3. They were also required to have psoriasis impacting between 3% to 10% of their body area.
On average, participants had spent more than 2 years being treated with secukinumab or ixekizumab prior to the study.
At weeks 0, 4, and once every subsequent 12 weeks until the 52-week endpoint (without a washout period), participants received 150 mg of Skyrizi. Researchers sought an endpoint of 0 to 1 on the sPGA scale by week 16 of the study.
Secondary endpoints included an sPGA score of 0 by the study’s conclusion, a Dermatology Life Quality Index (DLQI) score of 0 to 1 at weeks 16 and 52, and a Psoriasis Symptom Scale (PSS) score of 0, also at weeks 16 and 52.
At week 16, 19.8% of participants achieved a sPGA score of 0.
By the study’s conclusion, 63% of participants had achieved an sPGA score of 0 or 1, indicative of clear or almost clear skin. Additionally, 26.2% had achieved an sPGA score of 0.
Researchers did not note any new, additional safety signals to result from the study, but assessed participant safety throughout.
Study limitations included a lack of a vehicle-control group and potential variance in the definition of a suboptimal IL-17A response.
In a recent study of a similar nature, researchers found that risankizumab was effective in treating patients with plaque psoriasis who had a history of biologic use. The phase 3 study spanned 256 weeks and included a placebo group and measured participant progress using the Psoriasis Area and Severity Index (PASI). At week 256, a range of 72.7% to 87.5% of participants with a history of biologic use had achieved a PASI score of 90.
“Advanced therapies represent an important option in the treatment of plaque psoriasis, but as a physician, it's critically important to continually assess if patients are having an optimal response to treatment, as residual psoriasis can still have a significant impact on a patient's life," said Richard Warren, BSc, MBChB, MRCP, PhD in the press release. Warren is a professor from the University of Manchester and Norten Care Alliance in the United Kingdom.
“This study showed that risankizumab was able to improve clinical signs and symptoms of patients who had a suboptimal response with the anti-IL-17 therapies secukinumab and ixekizumab, contributing to the whole of evidence supporting risankizumab use in moderate to severe plaque psoriasis,” he shared.
Reference
- Abbvie announces late-breaking results of study evaluating 52-week efficacy and safety of SKYRIZI® (risankizumab) in plaque psoriasis patients with a prior suboptimal response to IL-17 inhibitor therapy. News Center. https://news.abbvie.com/news/press-releases/abbvie-announces-late-breaking-results-study-evaluating-52-week-efficacy-and-safety-skyrizi-risankizumab-in-plaque-psoriasis-patients-with-prior-suboptimal-response-to-il-17-inhibitor-therapy.htm?view_id=4667. Published March 18, 2023. Accessed March 24, 2023.

Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.