- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Article
RNAi compound targets connective tissue growth factor to reduce scar formation
Dermatologists discuss the challenges of treating hypertrophic scars and how a treatment in early trials might make post-treatment recurrence less likely.
Hypertrophic scars as a result of surgical incisions are difficult to predict, prevent and treat. And despite an abundance of treatment options, the scars often recur.

Dr. Mamelak“I have patients present with thick, symptomatic or unsightly scars, often not fully knowing the details of how these came about. It is sometimes difficult to determine if these scars form because of an underlying genetic predisposition or technical issues related to their surgery and aftercare,” says Austin, Texas, dermatologist and Mohs surgeon Adam Mamelak, M.D.
“This is important because when we select treatments to improve the appearance of scarring we can modify the technical aspects of our treatments; however, it is much more difficult to modify a genetic predisposition. Overall, we want to improve the appearance of the scar with the treatments, not simply replace one hypertrophic scar with another, or even make it worse.”
Treatment challenges
New York, N.Y., dermatologist Jeannette Graf, M.D., says it’s difficult to predict which patients will get hypertrophic scars, making the scarring a challenge to prevent.
But even when the scars mar body parts that can be easily covered by clothing, hypertrophic scars can be uncomfortable, itchy, even painful, which sends many patients to their dermatologists seeking solutions.
Another problem is that even when they’re treated, hypertrophic scars often are recurrent, according to Dr. Graf.

“…[W]hen someone gets a hypertrophic scar, particularly on certain areas of the body, like the chest, which is probably one of the most common areas to get them because the skin is so tight, they’re not predictable. People come in for injections (injectable steroids are really the mainstay of our treatment) and the scars go down and come back up. What doesn’t seem to get better with hypertrophic scars that recur is their thickness. So, you can flatten them but they stay kind of wide and thick,” Dr. Graf says.
Treatment options lack high-level evidence
Treatment options for the scars are numerous, according to Dr. Mamelak.
“The problem is the trials and level of evidence associated with these trials are sometimes questionable,” Dr. Mamelak says.
Silicone dressing and vitamin E are among the over-the-counter treatment options that people commonly use to hydrate and decrease inflammation associated with these scars. There also are a number of botanical and “natural” products, according to Dr. Mamelak.
The trouble with many of these, however, is research is lacking or the level of evidence is questionable, he says.
Topical treatments with onion skin extract, such as Mederma (Merz) and Contractubex (Merz) are popular and well-liked by patients who use them, according to Dr. Mamelak. Other options that don’t need a prescription include green tea, for its antioxidant and anti-inflammatory properties, and aloe vera, which is often used in wound healing but has not been formally evaluated for its effects on scar quality.
“Imiquimod cream and mitomycin C have also been used to help modify scarring, especially after keloid excision, with variable success in preventing recurrence,” Dr. Mamelak says. “Intralesional Kenalog is probably one of the most common treatments used for hypertrophic scars and keloids. It helps thin thickened scars, improves symptoms and increases pliability in stiff scar tissue. [Another common prescription treatment is] 5-fluorouracil. While it is effective, the pain associated with the injections and hyperpigmentation that often follows treatment can be limiting factors.”
A number of laser devices have also shown to be effective, according to the dermatologist.
“Pulse dye laser has been shown to improve scar pliability, appearance and color. Fractionated laser resurfacing has also been shown to help remodel scars,” he says. “Finally, in certain cases, scar revision where the hypertrophic scar is excised and the skin is repaired is often the most effective approach. The risk, however, is replacing one bad scar with another.”
Dr. Graf, who agrees that some of these treatments help, says there’s currently nothing that is definitely going to prevent hypertrophic scars from coming back.
NEXT: Something new
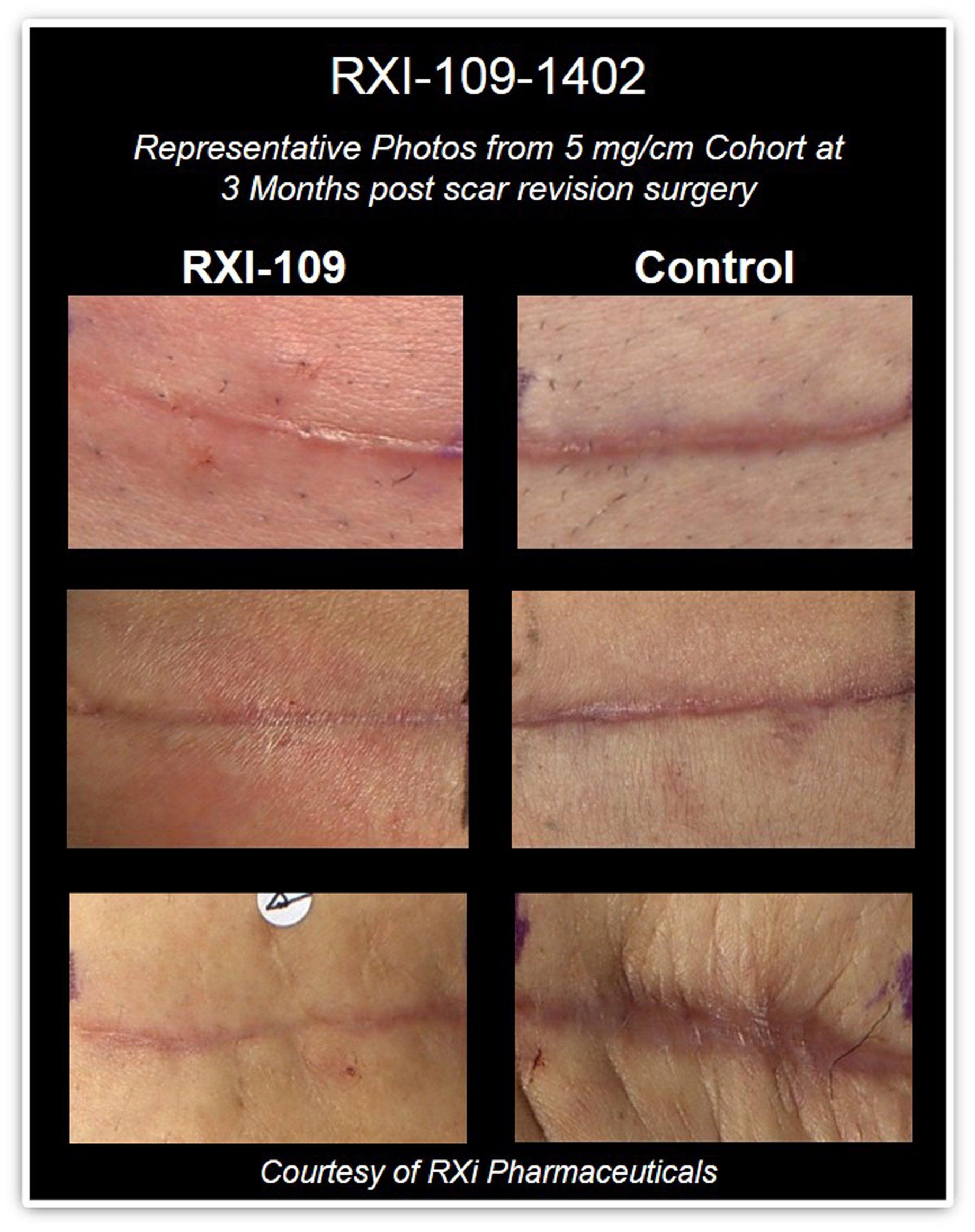
In initial cohorts, RXI-109 was administered by a regimen of intradermal injections over three months following scar revision surgery: RXI-109 treated (A) vs. untreated (B). Current cohorts are being treated over six months with additional doses to determine the best treatment regimen. Photos: RXi Pharmaceuticals
Room for something new
There’s a new technology, in phase 2 clinical studies, that aims to offer a different approach to reducing the unsightly scars after scar revision surgery. RXI-109 (RXi Pharmaceuticals) is a self-delivering messenger RNA interference technology, which reduces local connective tissue growth factor (CTGF) expression. CTGF regulates many of the biological pathways involved in fibrosis, including cutaneous scar formation, according to RXIPharma.com1.
Dr. Graf, who is a member of RXi’s scientific advisory board and advises on the company’s technology, says the findings, so far, are promising.
She says that CTGF, which is part of the wound-healing cascade, tends to be prolonged in patients who are likely to get hypertrophic scarring.
“In normal wound healing, CTGF is only present for a short period of time -just so the wound healing can occur. But if patients continue to heal because they have extended levels of connective tissue growth factor, they continue to over-heal, and then you get hypertrophic scars,” Dr. Graf says.
In their early studies looking at RXI-109 treatment for hypertrophic scars, researchers have found that the ideal time to start injecting the scars is at two weeks after surgery. Preliminary data suggest that subjects with surgically revised hypertrophic scars had less visible scarring after six doses of RXI-109 over the course of three months. But Dr. Graf says duration of treatment continues to be evaluated.
“We had fabulous results at three months, but, in those patients who had extra levels of connective tissue growth factor, we’re going to six months now, because we’re going to need to treat longer,” she says. “The beauty of this treatment is that it’s so specific. It does not interfere with anything else. It does not interfere with DNA. It interferes simply with the message being sent from the RNA to quiet down the connective tissue growth factor. And that’s how we can prevent hypertrophic scars.”
While researchers have seen the expected initial redness at the injection site and side effects common to a healing incision, most are mild to moderate side effects, according to Dr. Graf.
“The issue with this type of technology is getting it into the cell. [RXI-109] goes into the cell and is stable. There’s no carrying system. That has been the major factor of success in implementing these experiments,” Dr. Graf says.
RXI-109 is also being studied for macular degeneration, with risk of subretinal fibrosis. While the researchers still are at the cell culture level for other areas of dermatology, they’ve seen in other studies that RXI 231 inhibits tyrosinase, an enzyme responsible for melanin. Reduction of tyrosinase may improve the appearance of uneven skin tone, said Dr. Graf.
Dr. Graf says that if all goes well, the technology could continue into a larger phase 2 study in about a year.
Disclosures
Dr. Mamelak reports no relevant conflicts.
Dr. Graf serves on the scientific advisory board and is a researcher for RXi
References:
1 http://www.rxipharma.com/technology/rxi-109/