- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
Article
Sculptra stimulates cells to generate collagen
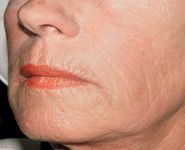
Tampa, Fla. — A new filler product unlike any available before is showing good results in correcting the signs of fat loss or lipoatrophy in the maturing face. The product called Sculptra? (Aventis) is an injectable implant that contains microparticles of poly-L-lactic acid, a biodegradable, synthetic polymer from the alpha-hydroxy-acid family.

Manufactured for Dermik Laboratories by Aventis Pharmaceuticals, Sculptra received approval by the U.S. Food and Drug Administration (FDA) in August 2004 for use in people with human immunodeficiency virus (HIV) to correct lipoatrophy due to medications. With the green light for use in the HIV population, this off-label use quickly followed.
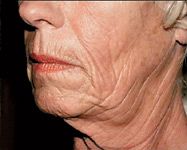
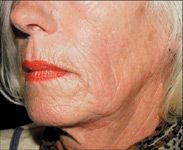
Sculptra requires a careful injection technique, preferably by a physician, according to Dr. Weinkle. "It's important that the product is injected at the proper plane, which is at the base of the dermis and into the subcutaneous layer," she says. "If you put it too superficially, there's a greater possibility of developing some nodules under the skin." A retrograde injection is required. Using the threading or tunneling technique, a thin trail of Sculptra should be deposited in the tissue plane as the needle is withdrawn.
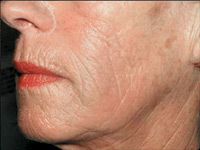
A key to properly evaluating patient results is to treat, wait and assess. Revolumizing results can take several weeks and patients should be evaluated no sooner than two weeks after the injection to determine if additional correction is needed, according to Dr. Weinkle. "My patients appreciate that the injection is painless," she says. "And, there's no downtime - patients can go right back to work or out to dinner that night."