- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
Dermatology Times
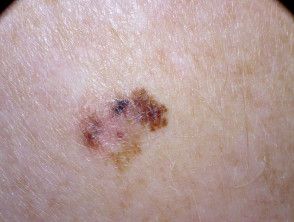
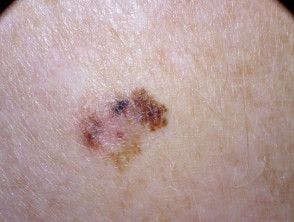
Skin Cancer Breakthroughs, Prevention, and Treatment Advances During the Past Year
Author(s):
This recap of skin cancer innovations accompanies the cover story of our May publication.
May is Skin Cancer Awareness Month.
The story from the cover of our May publication spotlights insights from a Florida clinic specializing exclusively in skin cancer care; pearls for treatment, awareness, and education; and more.
Below, we recap breakthroughs and prevention and treatment advances made in the realm of skin cancer from the past year. This recap accompanies our May cover story.
Steps Forward in Pharmaceuticals and Devices
In January, Medicus Pharma Ltd submitted a phase 2 IND clinical protocol to the FDA for a novel noninvasive treatment for basal cell carcinoma. The clinical protocol, SKNJCT-003, uses microarray needles containing doxorubicin.1 Verrica Pharmaceuticals’ phase 2 study of VP-315 [NCT05188729], a potential first-in-class oncolytic peptide-based immunotherapy for basal cell carcinoma, completed dosing of its last patient, the company announced in January.2
Lifileucel, which targets advanced melanoma, received accelerated approval from the FDA in February.3 In March, Dermatology Times spoke with Robert Den, MD, of Alpha Tau, a company exploring the potential of Alpha-DaRT, a new therapy that harnesses alpha particle–emitting atoms to specifically attack cancer cells while sparing healthy tissue, in skin cancer treatment.
A recent study found that the combination of dermoscopy with reflectance confocal microscopy enhanced the diagnostic accuracy of amelanotic/hypomelanotic lentigo maligna/lentigo maligna melanoma, aiding dermatologists in accurately identifying skin lesions and improving patient outcomes.4 Superficial x-ray and radiation therapy have also emerged as a well-tolerated nonsurgical option for basal and squamous cell carcinomas, offering patients an alternative treatment modality with minimal adverse effects, particularly for those who are not candidates for surgery.5-6
Artificial Intelligence and Advanced Tech Strides
A study published in January revealed that the integration of explainable artificial intelligence significantly improves clinician confidence and diagnostic accuracy in melanoma detection, leading to more accurate and timely interventions.7 Additionally, virtual reality (VR) proved to be a useful tool in dermatology education in one study, with a VR skin cancer screening scenario positively affecting the subjective competence of dermatology students, offering a technically feasible and immersive learning experience for medical professionals.8
Progress in Awareness and Research
DermTech’s 2-gene expression profiling (GEP) assay demonstrated success in guiding biopsy decision-making for ambiguous skin lesions across all skin phototypes. The noninvasive test offers clinicians a tool for determining whether further biopsy or treatment is necessary, reducing unnecessary procedures and patient anxiety. Dermatology Times spoke with Maral Kibarian Skelsey, MD, the author of the Trust 2 study, in January. The study validates DermTech’s 2-GEP assay’s high negative predictive value by assessing its ability to detect the expression of LINC00518 and PRAME.
Regarding awareness, Dermatology Times also spoke with the Skin Cancer Foundation to discuss its initiative, Destination Healthy Skin, which uses a recreational vehicle to travel to various cities across the country, where dermatologists volunteer to provide on-the-street, free-of-cost skin screenings. By bringing screening services to communities, the initiative aims to improve outcomes through early intervention.
References
- Medicus Pharma Ltd. submits to the FDA phase 2 IND clinical protocol to non-invasively treat basal cell carcinoma of the skin. News release. Medicus Pharma. January 3, 2024. Accessed April 29, 2024. https://medicuspharma.com/medicus-pharma-ltd-submits-to-the-fda-phase-2-ind-clinical-protocol-to-non-invasively-treat-basal-cell-carcinoma-of-the-skin/
- Verrica Pharmaceuticals announces last patient dosed in part 2 of phase 2 study of VP-315, a potential first-in-class oncolytic peptide-based immunotherapy, for the treatment of basal cell carcinoma. News release. BioSpace. January 5, 2024. Accessed April 29, 2024. https://www.biospace.com/article/releases/verrica-pharmaceuticals-announces-last-patient-dosed-in-part-2-of-phase-2-study-of-vp-315-a-potential-first-in-class-oncolytic-peptide-based-immunotherapy-for-the-treatment-of-basal-cell-carcinoma/
- Iovance’s AMTAGVI (lifileucel) receives US FDA accelerated approval for advanced melanoma. News release. Iovance Biotherapeutics. February 16, 2024. Accessed April 29, 2024. https://ir.iovance.com/news-releases/news-release-details/iovances-amtagvitm-lifileucel-receives-us-fda-accelerated
- Spadafora M, Megna A, Lippolis N, et al. Dermoscopy and reflectance confocal microscopy of solitary flat pink lesions: A new combined score to diagnose amelanotic melanoma. J Eur Acad Dermatol Venereol. April 4, 2024. doi:10.1111/jdv.19991
- Mattia A, Thompson A, Lee SK, Hong HG, Green WH, Cognetta Jr. AB. Superficial x-ray in the treatment of non-aggressive basal and squamous cell carcinoma in the elderly: A 22-year retrospective analysis. J Am Acad Dermatol (2024), https://doi.org/10.1016/j.jaad.2024.01.010
- Rezaei SJ, Eid E, Tang JY, Kurian AW, Kwong BY, Linos E. Incidence of nonkeratinocyte skin cancer after breast cancer radiation therapy. JAMA Netw Open. 2024;7(3):e241632. doi:10.1001/jamanetworkopen.2024.1632
- Chanda T, Hauser K, Hobelsberger S, et al. Dermatologist-like explainable AI enhances trust and confidence in diagnosing melanoma. Nat Commun. 2024;15(1):524. January 15, 2024. doi:10.1038/s41467-023-43095-4
- Junga A, Schmidle P, Pielage L, et al. New horizons in dermatological education: Skin cancer screening with virtual reality. J Eur Acad Dermatol Venereol. March 18, 2024. doi:10.1111/jdv.19960

Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.