- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Publication
Article
Dermatology Times
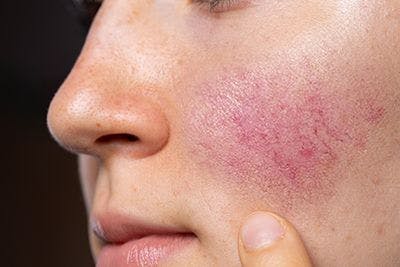
Specialized skincare regimen improves rosacea symptoms
Author(s):
Effects of a specialized skincare regimen for sensitive and redness-prone skin were investigated in an eight-week, prospective, controlled clinical trial enrolling 60 patients with rosacea. The intervention was associated with improvements in objectively measured erythema, rosacea symptoms, and health-related quality of life.
Patients with rosacea using a set of skincare products formulated for sensitive and redness-prone skin benefited with improvements in disease-related signs and symptoms as well as quality of life, reported researchers in a study published online in the Journal of Cosmetic Dermatology.1
The skincare regime was comprised of three commercially available products (LETISR sensitive and red skin), including micellar water for cleansing in the morning and evening, a broad-spectrum (UVA/UVB) SPF20 cream, and night serum.
At the end of the eight-week, prospective, randomized study, patients using the specialized skincare products experienced greater relief of rosacea symptoms, reduction of objectively measured erythema, and improvement in the Dermatology Life Quality Index (DLQI) score compared with controls who continued to use their previous skincare products.
RELATED: Pilot study examines facial microbiome diversity
The investigators observed that the findings are strengthened by the prospective controlled design of their study and its use of an objective method to quantitate facial erythema. Commenting further, they state “With countless available skincare products, in vivo studies with objective evaluation methods are necessary in order to prove their efficacy.”
The study was conducted by members of the Department of Dermatology and Allergy, University Hospital Munich, Munich, Germany, and enrolled 60 Caucasian patients (44 women, 16 men) with rosacea who had persistent centrofacial erythema. Eligible patients were required to have received no treatment for the redness within the past three months.
The participants were randomized 5:1 to the intervention and control groups. Patients in the intervention group received precise instructions on the use of the study products. All patients were told to avoid excessive sun exposure and use of other rosacea treatments.
Safety and efficacy data were collected at four and eight weeks after the baseline visit. Facial erythema was evaluated as the primary endpoint and was determined by analysis of the intensity of the color red at five defined points per cheek on images obtained with a three-dimensional imaging device (VECTRA, Canfield Scientific). The investigator performing the measurements was blinded to the patients’ study group assignment.
Patients also completed both the DLQI and a symptom questionnaire at baseline and each follow-up. The symptom questionnaire assessed the presence of itching, tension, warmth, burning, and dryness.
Of the 60 enrolled patients, 44 (73%) completed the eight-week study. Thirteen of the 16 patients who did not complete the study were lost to follow-up and all were in the intervention group. Two patients in the intervention group withdrew early due to mild worsening of facial redness and pustules accompanied by a burning sensation. Treatment adherence in the intervention group, which was determined by weighing the returned skincare products at study completion, was considered to be good.
The analyses of facial erythema showed a statistically significant improvement after just four weeks in both study groups. However, only the intervention group had a statistically significant further improvement over the next four weeks. At study completion, facial erythema was reduced from baseline by 16% in the intervention group and 8% in the control group. The investigators commented that the continuous improvement observed in the intervention group might indicate the superior efficacy of the study products.
Overall, the patients participating in the study had mild symptoms at baseline. The mean total symptom score in the intervention group improved by 28.6% after four weeks and by 57.1% after eight weeks. Both changes were statistically significant, and at eight weeks, it was noted that symptoms in the intervention group were nearly fully diminished. There was no change from baseline in mean total symptom score in the control group at either follow-up visit.
Mean baseline DLQI scores were 3.3 in the intervention group and 2.1 in the control group, indicating that the patients in the study had a relatively high overall quality of life. Nevertheless, statistically significant reductions in DLQI score (indicating improvement) were achieved in the intervention group at both 4 weeks (-51.5%) and 8 weeks (-54.5%), and the change was attributed to improvement in clinical appearance. Relative to baseline, the mean DLQI score in the control group decreased by 4.8% at 4 weeks and by 23.8% at eight weeks, but neither change was statistically significant.
The two patients in the intervention group who withdrew from the study because of side effects experienced slight aggravation of erythema and pustules along with burning. The patients declined patch testing to investigate the cause, and so the investigators stated that an association with use of the skincare products could not be established.
Disclosure:
The study was funded by the manufacturer of the skincare products used in the intervention group. One of the authors disclosed receiving fees as a speaker and advisor to LETI Pharma and other companies that market pharmaceuticals and cosmeceuticals for rosacea.
Reference:
1 Guertler A, Jøntvedt NM, Clanner-engelshofen BM, Cappello C, Sager A, Reinholz M. Efficacy and safety results of micellar water, cream and serum for rosacea in comparison to a control group. J Cosmet Dermatol. 2020; doi: 10.1111/jocd.13591

Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.