- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Publication
Article
Dermatology Times
Topical minocycline foam safe, well-tolerated
Author(s):
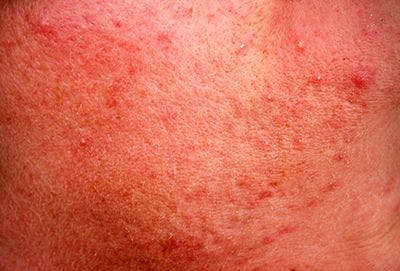
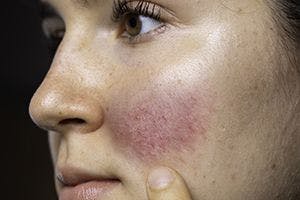
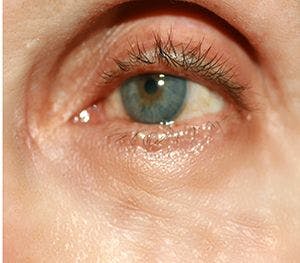
A topical minocycline foam application appears effective and was well-tolerated by patients with moderate-to-severe papulopustular rosacea.
Daily topical minocycline foam appears to be safe, was well-tolerated and did not result in adverse events according to pooled data presented during the AAD VMX Virtual Meeting Experience 2020, June 12-14.1
“Topical therapies such as metronidazole, azelaic acid, and ivermectin are considered first-line therapies for papulopustular rosacea. Oral tetracyclines, specifically doxycycline and minocycline, are mainstays of treatment for moderate-to-severe disease; however, they are associated with significant systemic side effects,” writes Linda Stein Gold, M.D., director of dermatology clinical research, Henry Ford Health System, Detroit, and colleagues in the poster.
The researchers examined data from a pooled analysis of two identical phase 3, randomized, double-blind, vehicle-controlled studies that investigated efficacy, safety and tolerability of FMX103 1.5% topical minocycline foam in patients with papulopustular rosacea.2
The studies included a total of 1,521 patients (mean age: 50 years) who were randomized 2:1 to receive FMX103 1.5% or vehicle once daily for 12 weeks.
Data showed that 341 (22.4%) patients reported treatment-emergent adverse events (TEAE). The most frequently reported TEAEs in the FMX103 1.5% vs vehicle groups, respectively, included viral upper respiratory tract infection (2.4% vs 2.3%), upper respiratory tract infection (1.9% vs 2.5%) and headache (1.4% vs 1.9%). The majority of the TEAEs reported by patients were mild in severity. No differences were observed between the treatment groups in the incidence of TEAEs. At week 12, the researchers found that all facial tolerability assessments had higher percentages of “none” compared to baseline and trended toward improving scores in the FMX103 1.5% treatment group.
For patients with moderate-to-severe papulopustular rosacea, FMX103 1.5% minocycline foam not only proves to be effective but a favorable safety profile could be maintained during the studies. As such, the novel foam application could represent a viable treatment alternative to current standard oral treatment regimens for patients with more advanced papulopustular rosacea disease.
Disclosures
Dr. Stein Gold is an advisor and investigator for Foamix, Galderma, LEO Pharma, Novartis, and Valeant and is an investigator for Janssen, AbbVie, and Solgel.
References
1. Gold LS, Del Rosso JQ, Kircik L, Bhatia ND, Hooper D, Nahm W, Stuart I. Integrated Safety Analysis of FMX103 1.5% Topical Minocycline Foam for the Treatment of Moderate-to-Severe Papulopustular Rosacea (PPR): Results from Two Phase 3 Studies. E-poster presented at AAD VMX Virtual Meeting Experience 2020 July 12-14. https://pubmed.ncbi.nlm.nih.gov/32004648/
2. Gold LS, Del Rosso JQ, Kircik L, et al. Minocycline 1.5% foam for the topical treatment of moderate to severe papulopustular rosacea: Results of 2 phase 3, randomized, clinical trials. J Am Acad Dermatol. 2020;82(5):1166-1173.

Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.