- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
Trifarotene cream proves safe, effective for acne vulgaris
Author(s):
A recent clinical review proves trifarotene 0.005% cream is an effective and safe treatment for acne vulgaris on the face and neck in patients 9 years and older. Currently, the drug is one of four topical retinoid acne treatments available on the market.
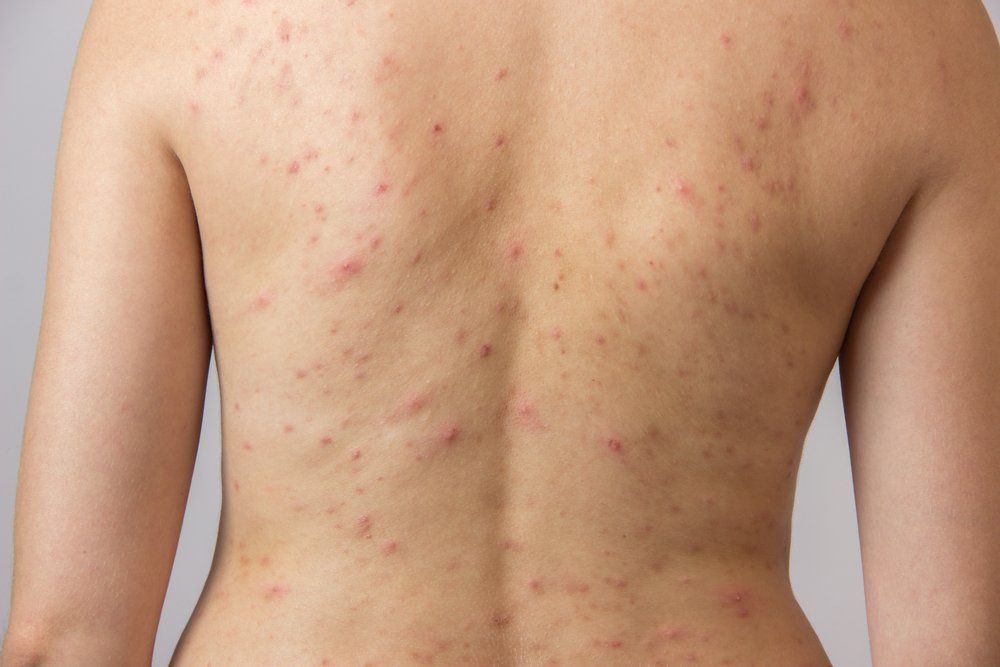
The U.S. Food and Drug Administration (FDA) approval of trifarotene 0.005% cream (Aklief, Galderma) in October 2019 brought to market the first new topical retinoid for the treatment of acne in more than two decades and offered physicians a unique and highly targeted way to reduce lesions on the face and trunk.
Trifarotene cream, which is approved for acne vulgaris in patients 9 years and older, is currently one of four FDA-approved topical retinoids on the market to treat acne including tretinoin, adapalene (Differin, Galderma) and tazarotene. Retinoids, which bind to the retinoic acid receptor (RAR), have three isoforms: RAR-alpha, RAR-beta and RAR-gamma. Trifarotene is unique because it is the only topical retinoid that selectively targets RAR-gamma, which is the most common RAR found in the skin, and it is the first topical treatment studied and proven to treat both facial (forehead, cheeks, nose and chin) and truncal (chest, shoulders and back) acne, according to the company.1
MORE: An update on antibiotic use for acne
Efficacy and safety of trifarotene was established by data from two pivotal phase 3 clinical trials (PERFECT 1 and PERFECT 2), in which a total of 2,420 patients 9 years and older with moderate truncal and facial acne were enrolled. Patients were randomized to receive trifarotene 0.005% cream or vehicle once daily before bed, according to a clinical review published by The Medical Letter in which authors discussed the efficacy, safety and tolerability findings.2
As primary endpoints, researchers evaluated for facial clearance success as determined by a ≥2-grade improvement on the Investigator’s Global Assessment (IGA) scale as well as absolute change from baseline in noninflammatory and inflammatory lesion counts at week 12. Secondary endpoints included evaluation of truncal lesion clearance success as determined by a ≥2-grade improvement on the Physician’s Global Assessment (PGA) scale as well as absolute change from baseline to week 12 in noninflammatory and inflammatory lesions counts.3
Trifarotene met all primary and secondary endpoints in both trials, demonstrating a significant improvement in treatment success rates for facial and truncal acne with 29.4% of patients achieving IGA success with trifarotene in PEFECT 1 versus 19.5% with vehicle and 42.3% vs. 25.7% in PERFECT 2. PGA success was recorded for trifarotene in truncal acne with 35.7 versus 25 for vehicle (PERFECT 1) and 42.6 trifarotene versus 29.9 vehicle (PERFECT 2).2
Also, data from the trials exhibit a 19- and 25-point reduction in inflammatory and non-inflammatory lesion counts for the face in PERFECT 1 (compared to -15.4 and -17.9 in vehicle), and a reduction of 24.2 and 30.1 in PERFECT 2.
Meanwhile, results for the drug’s effects on the trunk include a reduction of inflammatory and noninflammatory lesion counts is -21.4 and -21.9 in PERFECT 1 (versus -12.8 and -17.8 vehicle), and -25.5 and -25.9 in PERFECT 2 (versus -19.8 and -20.8 vehicle).
Adverse events were mild to moderate, with the most common being application site-irritation and pruritus generally peaking at four weeks and then declining.
Sunburn (2.6% versus 0.5% with vehicle), irritation at application site (7.5% versus 0.3%) and application-site pruritus (2.4% versus 0.8%) were some of the most commonly reported adverse events in both of the trials with effects diminishing after the first 4 weeks of usage. Trifarotene also exhibited the typical tolerability profile of a topical retinoid ranging from mild to moderate but manageable.2
RELATED: Treatment options for acne expand
In conjunction with the two 12-week trials, another extended single-arm study was conducted with 453 patients over the course of 52 weeks. Researchers observed treatment success of 38.6% for the trunk and 26.6% for the face by week 12. By week 52, treatment success rates rose to 65.1% for face and 66.9% for the trunk. Additionally, 22.6% of patients reported acne had no impact on their quality of life (QoL) at baseline and 53.8% reporting no impact by week 52.3
Since its approval by the FDA in October 2019, trifarotene provides another viable treatment option for physicians to prescribe to their patients that can treat acne vulgaris effectively and safely on the face and trunk. The authors note while the trials prove trifarotene is safe and effective, it is on the expensive end for treatment options and can cause skin irritation.
References:
1. Trifarotene (Aklief) - A New Topical Retinoid for Acne. Access to Article | The Medical Letter, Inc. https://secure.medicalletter.org/article-share?a=1587a. Published December 16, 2019. Accessed May 7, 2020.
2. Tan J, Thiboutot D, Popp G, et al. Randomized phase 3 evaluation of trifarotene 50 μg/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol. 2019;80(6):1691-1699.
3. Blume-peytavi U, Fowler J, Kemény L, et al. Long-term safety and efficacy of trifarotene 50 μg/g cream, a first-in-class RAR-γ selective topical retinoid, in patients with moderate facial and truncal acne. J Eur Acad Dermatol Venereol. 2019;
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.












