- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
Tyrosine Kinase 2 inhibitors: A phase 2 trial in psoriasis
Author(s):
Researchers recently performed a control trial to test the efficacy of BMS-986165 a potent TYK2 inhibitor. Here's what they found.
Researchers recently performed a control trial to test the efficacy of BMS-986165 a potent TYK2 inhibitor. Here's what they found.
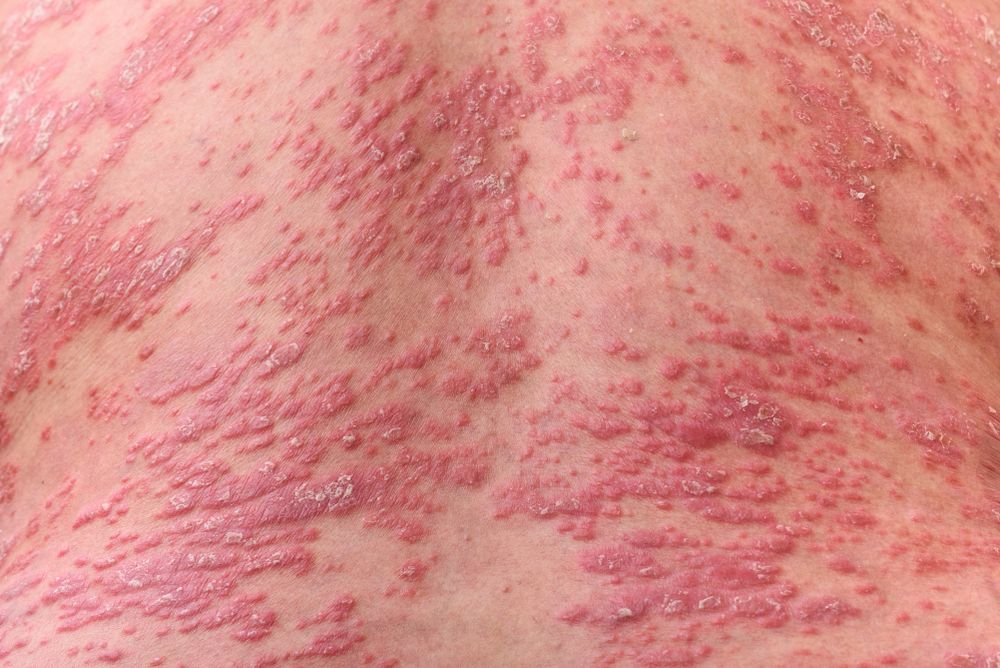
Psoriasis patients suffer from systemic inflammation manifesting as skin lesions. It is well established that both interleukin-12 (IL-12) and IL-23 are involved in the inflammatory cascade and can be antagonized via treatments with monoclonal antibodies.
Tyrosine Kinase 2 (TYK2) inhibitors represent another potential modality that can manipulate these inflammatory cytokines. The mechanism of action of TYK2 inhibitors is via inhibition of the STAT dependent pathway leading to a decrease in the production of IL-12 and IL-23.
Dr. Papp and colleagues performed an international, multicentered, double-blind randomized control trial to test the efficacy of BMS-986165 a potent TYK2 inhibitor. The study was published in the New England Journal of Medicine, September of 2018.
The primary endpoint of the trial was a PASI score reduction of 75% or greater from baseline. The secondary endpoint was the evaluation of adverse events in patients receiving BMS-986165 (TYK2).
Patients were randomly assigned into groups divided by dosing of the test drug. The groups were divided as follows: placebo, 3 mg every other day, 3 mg daily, 3 mg twice daily, 6 mg twice daily, and 12 mg daily. Patients were stratified based on geographic region (Japan or the rest of the world) and previous treatment with a biologic agent (yes or no). Patients, investigators, and the sponsors were blinded to the trial-group assignments.
The results showed that BMS-986165 was more effective than placebo. There appears to be a dose response with the 12 mg daily dosing of TYK2 achieving a PASI 75 in 70% of participants at 12 weeks compared to 4% in placebo. Patients in the 12 mg daily dose who were naive to a biologic agent achieved a PASI 75 of 81% compared to 4% in the placebo group.
Adverse events were also reported in the trial. The most common adverse event reported was nasopharyngitis followed by a headache. There were no changes in vital signs, ECG, or lab values in the treatment groups.
This trial showed similar results to the IL-12, IL-23, and anti-TNF trials compared to placebo.
The authors acknowledge, however, the limitations of this study which include small sample size and short duration of the study. BMS-986165 will require a longer study to test the medications durability as well as a larger cohort to test the safety of this agent.
Reference:
Papp, Kim, et al. "Phase 2 trial of selective tyrosine kinase 2 inhibition in Psoriasis." New England Journal of Medicine 379.14 (2018): 1313-1321.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.







