- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
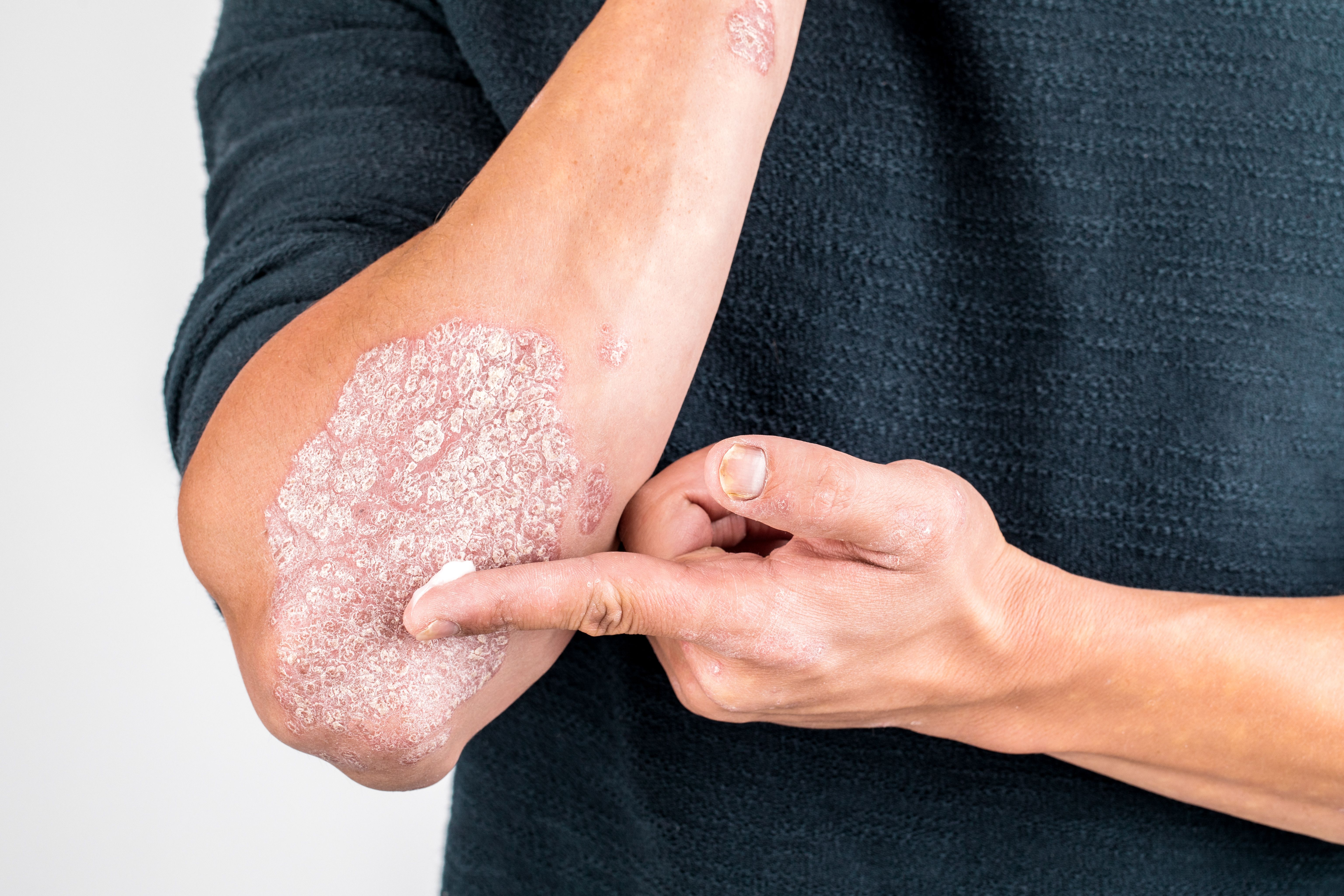
Abatacept: Pricey, but Effective
Author(s):
The approval of abatacept (Orencia/Bristol-Myers Squibb) for psoriatic arthritis (PsA) earlier this month by the FDA offers a new treatment option for patients who are struggling to keep the disease under control.

The approval of abatacept (Orencia/Bristol-Myers Squibb) for psoriatic arthritis (PsA) earlier this month by the FDA offers a new treatment option for patients who are struggling to keep the disease under control.
Abatacept is a T-cell inhibitor and is the first of its kind for PsA. It has proven to be a successful treatment option, but it is also one of the most expensive treatments for PsA.
The last major advancement in new treatments for PsA was over 10 years ago with the development of TNF inhibitors, which were groundbreaking at the time. “A significant proportion of patients achieved clinical responses with around 40-50% reaching minimal disease activity states over time. However, relapse and/or loss of response is common and in a disease of relatively young age of onset requiring treatment over decades, there remains considerable unmet need,” wrote Iain B. McInnes of the University of Glasgow in the March 8, 2016 online issue of Clinical and Experimental Rheumatology.
T-cell inhibitors like abatacept have the potential to meet that unmet need for patients for whom TNF inhibitors or other biologics are not effective, or for patients who cannot tolerate adverse effects associated with other treatments.
However, the cost may present some challenges for patients seeking access to this new treatment. In this slideshow, we summarize how abatacept works, its adverse effects, how it differs from other biologics, its cost and other factors you should know about abatacept for psoriatic arthritis.
Citations































References:
1) Mease PJ, Gottlieb AB, van der Heijde D, et al. “Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase III study in psoriatic arthritis.” Annals of the Rheumatic Diseases. Published online first: 04 May 2017. DOI: 10.1136/annrheumdis-2016-210724
2) Strand, V., Balsa, A., Al-Saleh, J. et al. "Immunogenicity of Biologics in Chronic Inflammatory Diseases: A Systematic Review." BioDrugs (2017). DOI:10.1007/s40259-017-0231-8
3) Agnes Szentpetery, Eric Heffernan, Martina Gogarty, et al. "Abatacept reduces synovial regulatory T-cell expression in patients with psoriatic arthritis." Arthritis Research and Therapy. July 5, 2017. DOI: 10.1186/s13075-017-1364-3
4) FDA prescribing label for Abatacept (Orencia/Bristol-Myers Squibb).
5) Bristol-Myers Squibb press release.
6) “Targeted Immune Modulators for Rheumatoid Arthritis: Effectiveness & Value Evidence Report," Institute for Clinical and Economic Review (ICER)," April 7, 2017.
7) Iain B. McInnes. "Psoriatic arthritis: embracing pathogenetic and clinical heterogeneity?" Clinical and Experimental Rheumatology. March 8, 2016. Vol.34, N°4,Suppl.98 - PI 0009, PF 0011

Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.