- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Video
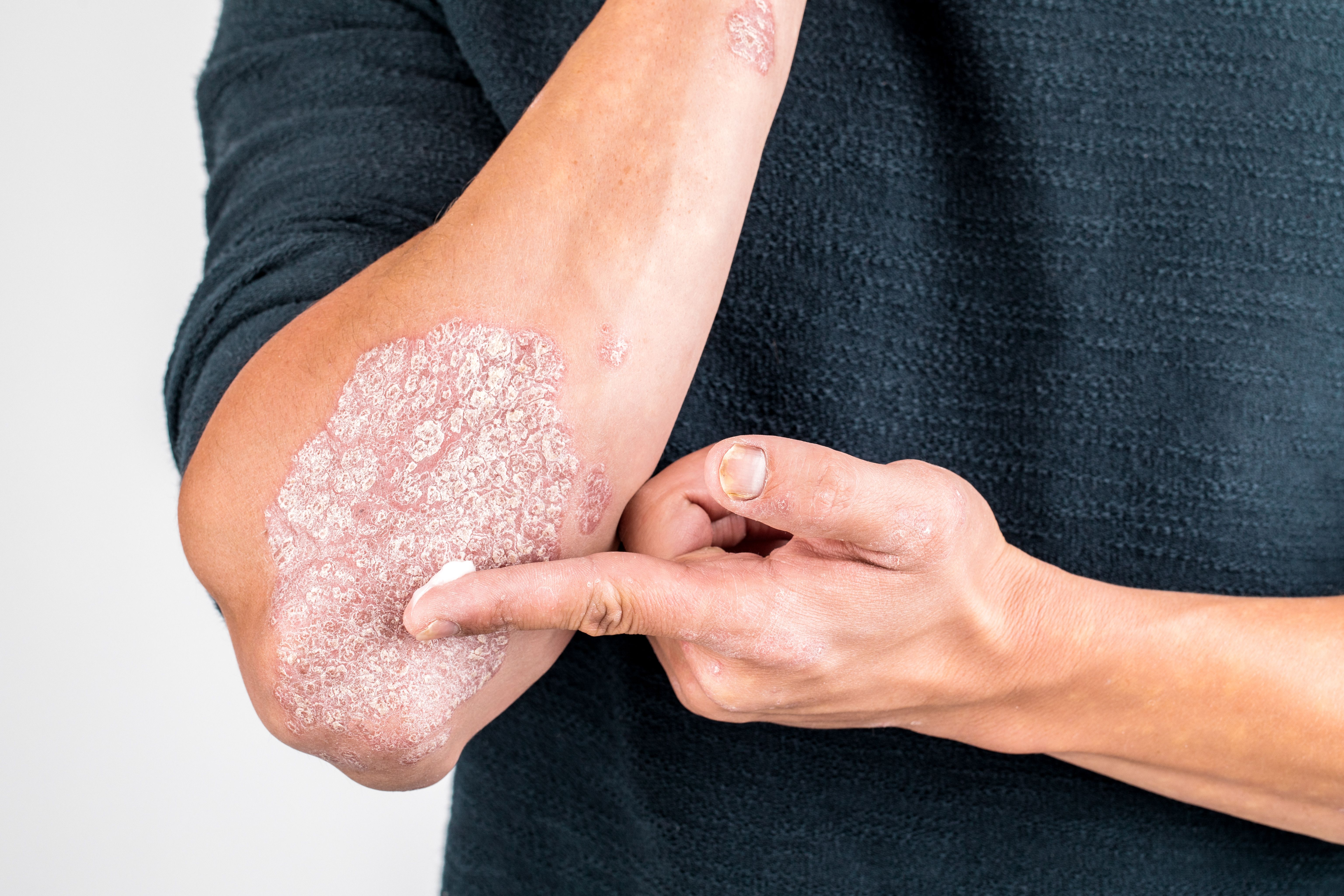
Bimekizumab’s Potential in Moderate-to-Severe Plaque Psoriasis
Author(s):
A focused discussion on the benefit of bimekizumab, an IL-17A/IL-17F inhibitor, in the setting of moderate to severe plaque psoriasis.
Transcript:
Christopher G. Bunick, MD, PhD: In thinking about all these biologic agents, in my research lab, we’ve looked at some of the molecular differences of the epitopes that these drugs bind. It’s quite interesting, even within a class. I did a Cutaneous Connection podcast for Dermatology Times®, No. 31. In that podcast, I reviewed some of that research, but I also touched on the new IL-17A/IL-17F inhibitor, bimekizumab, which Dr [Helen] Torok just touched on. Dr Lebwohl, you mentioned this early in the discussion, but what’s your viewpoint on the potential role for this IL-17A/IL-17F inhibitor, bimekizumab, in terms of the psoriasis treatment landscape?
Mark G. Lebwohl, MD: It’s an extraordinary drug. First, I could be wrong because it’s not approved in the United States, but I’d expect no boxed warnings because there were no serious adverse effects. There were mild to moderate yeast infections that occurred at a greater frequency than with the other IL-17 blockers, where we might expect to see yeast infections in 5% of patients. It starts to approach 20% of patients, but its 1 or 2 yeast infections aren’t recurrent. It’s 1 or 2 infections over years, and they’re very easy to treat. Few patients dropped out because of yeast infections. That’s the 1 drawback compared with the other IL-17 blockers. But they are among the fastest and strongest. When I say strongest, it’s the only treatment we’ve had where at the primary end point, the majority of patients achieved PASI [Psoriasis Area and Severity Index] 100. No other treatment has done that.
In addition, they’re surprisingly long-lasting. You can get few injections. In the trial, it was given every 4 weeks for 16 weeks and then they broke into 2 groups. One group got it every 4 weeks while the other got it every 8 weeks. There was no difference in response. It looks like we’ll have a drug that is fast, gives a durable response, and can be dosed as infrequently as every 2 months. Why does that occur? We’re not just blocking IL-17A, which has a major role in psoriasis, but also blocking IL-17F, which I would call nature’s backup to IL-17A. If something goes wrong with your IL-17A, you’re not left without IL-17 protection. IL-17F is more prevalent in psoriasis plaques than IL-17A is. Although IL-17A is obviously the key driver, there’s some contribution to the IL-17 effect by IL-17F. By blocking both, you get an additive benefit.
Christopher G. Bunick, MD, PhD: Yes, the additive benefit is important because there are studies showing synergy there and people weren’t able to necessarily predict that you’d see this additive effect. It might have been the IL-17A or the IL-17F, but the additive benefit may be why we’re seeing PASI 100 levels in this clinical trial.
Mark G. Lebwohl, MD: Right.
Christopher G. Bunick, MD, PhD: With regard to the candidiasis, it’s important for us to understand that all medicines have the potential for that adverse effect. If I’m not mistaken, Dr Lebwohl, didn’t some of the other IL-17 inhibitors also have lower levels of candidiasis in their trials?
Mark G. Lebwohl, MD: Yes. Those numbers approach 5%, but that’s nowhere near the numbers we’re seeing with bimekizumab.
Christopher G. Bunick, MD, PhD: I find it interesting that there are 1 or 2 episodes in treatment, and it’s not a chronic recurrent candidiasis. Is that a fair statement?
Mark G. Lebwohl, MD: No question about it. The vast majority were 1 or 2 episodes, and that’s it.
Christopher G. Bunick, MD, PhD: That’s very good.
Transcript edited for clarity.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.