- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
Dermatology Times
Considerations for Cardiovascular Risk in Patients With Psoriasis
Author(s):
Patients with psoriatic disease face an increased risk of cardiovascular disease. Clinicians should consider this when determining management and treatment modalities for patients with psoriasis.
SciePro-stock.adobe.com

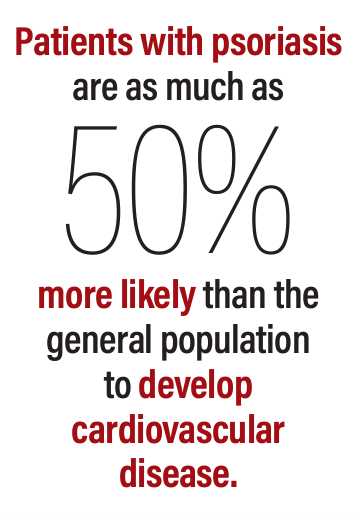
Patients with psoriasis are as much as 50% more likely than the general population to develop cardiovascular disease (CVD). Moreover, the risk of CVD shares a linear relationship with psoriatic disease severity.1
This relationship is further compounded by the notion that although cardiovascular risk factors and CVD are heightened among patients with psoriasis, psoriasis in itself is an independent risk factor. Furthermore, psoriasis is also an independent risk factor for myocardial infarction, and the risk of myocardial infarction, stroke, and death caused by cardiovascular events is at its greatest among this patient population.2
A recent study3 published in the Journal of Clinical Medicine explored the relationship between the new onset of several conditions in patients with moderate to severe psoriasis exposed to biologics. These conditions included major cardiovascular events, inflammatory bowel disease (IBD), oral and gastrointestinal (GI) candidiasis, and herpes zoster, among others.
In the comprehensive, multicenter, retrospective cohort study conducted across 16 hospitals in Korea, researchers analyzed real-world clinical data from an extensive cohort of 19,321,164 patients. The study focused on evaluating the safety profiles of biologics in the treatment of moderate to severe psoriasis, utilizing deidentified patient-level electronic health record data.
The study targeted 16 databases encompassing patients from various hospitals, with a collective sample size of 19,321,164 individuals. The investigation was conducted using the Observational Medical Outcomes Partnership Common Data Model (OMOP-CDM) platform and FeederNet, which is a Korean health data platform based on OMOP-CDM.
The target cohort included patients with moderate to severe psoriasis treated with biologics. The study defined specific inclusion criteria, such as a diagnosis of psoriasis, the prescription of at least 1 injection of the study drug, and a continuous observational period of more than 30 days before the first prescription. The study drugs comprised tumor necrosis factor-α inhibitors, IL-12/23 inhibitors, IL-17 inhibitors, and IL-23 inhibitors, approved for treating moderate to severe psoriasis in Korea.
The primary outcome was the incidence of adverse events in the target cohort. Adverse events of interest included new-onset IBD, oral/GI candidiasis, pulmonary tuberculosis, herpes zoster, and major adverse cardiovascular events. Comorbid diseases such as psoriatic arthritis, hypertension, type 2 diabetes mellitus, and hyperlipidemia were also considered.
The study included 2085 patients, with a mean age of 45.6 ± 13.4 years and a male-to-female ratio of 1.86. The baseline characteristics of patients treated with different biologics were analyzed.
The use of biological therapies, including IL-17 inhibitors, in patients with moderate to severe psoriasis is associated with a reduction in high-risk coronary plaque phenotypes, as revealed by coronary computed tomography and angiography–based evaluations.3
A post hoc analysis of pooled data from phase 3/4 secukinumab studies in patients with psoriasis and PsA demonstrated early and sustained reductions in inflammatory biomarkers predictive of CVD and CVD-related mortality. These biomarkers included C-reactive protein levels and the neutrophil-to-lymphocyte ratio, with improvements observed as early as week 12 and lasting over 52 weeks.
Additionally, endothelial function measured by flow-mediated dilation significantly improved in patients with moderate to severe plaque psoriasis treated with secukinumab for 52 weeks. However, low plasma IL-17A levels were associated with an increased incidence of CVD in patients with moderate to severe psoriasis.
Although there was no beneficial effect on aortic vascular inflammation, an imaging biomarker of CVD risk in patients treated with secukinumab, clinical trials did not find a significant association between IL-17A blockade and incidences of MACEs.
“IL-17 could play a role in the development of CVD, as indicated by the high mortality rate and recurrence of acute myocardial infarction in patients with low serum IL-17 levels,” study authors wrote. “However, whether IL-17 blockade has a beneficial effect on CVD risk in patients with psoriasis remains uncertain.”
References
- Garshick MS, Ward NL, Krueger JG, Berger JS. Cardiovascular risk in patients with psoriasis: JACC review topic of the week. J Am Coll Cardiol. 2021;77(13):1670-1680. doi:10.1016/j.jacc.2021.02.009
- Gisondi P, Bellinato F, Girolomoni G, Albanesi C. Pathogenesis of chronic plaque psoriasis and its intersection with cardio-metabolic comorbidities. Front Pharmacol. 2020;11:117. doi:10.3389/fphar.2020.00117
- Kang DH, Lew BL, Kwon SH. Incidence of new-onset inflammatory bowel disease, oral and gastrointestinal candidiasis, herpes zoster, pulmonary tuberculosis, and major cardiovascular events in patients with moderate-to-severe psoriasis exposed to biologics. J Clin Med. 2023;12(24):7653. doi:10.3390/jcm12247653

Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.