- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Publication
Article
Dermatology Times
Deucravacitinib Safe, Effective for Psoriasis
Author(s):
Deucravacitinib meets primary end points for treating moderate to severe psoriasis.
In 2 recent phase 3 trials, deucravacitinib (BMS-986165, Bristol Myers Squibb [BMS]), demonstrated progress toward becoming a new treatment for moderate to severe plaque psoriasis.1,2
Deucravacitinib is the first and only tyrosine kinase 2 (TYK2) inhibitor in clinical studies across multiple immune-mediated diseases, according to Mary Beth Harler, MD, senior vice president and head of immunology and fibrosis development, global drug development, at Bristol Myers Squibb in Princeton, New Jersey.
“The impetus for our research came from genetic analysis in human populations that revealed the small subset of individuals with impaired TYK2 function were actually protected from certain immune-mediated diseases, such as psoriasis, without an increased risk of significant complications,” she told Dermatology Times®.
BMS scientists designed deucravacitinib to selectively target TYK2, thereby inhibiting signaling of IL-12, IL-23, and Type 1 IFN, which are key cytokines involved in psoriasis pathogenesis, Harler said.
“Deucravacitinib achieves a high degree of selectivity for TYK2 by uniquely binding to its regulatory domain, locking it in an inactive state,” she said. “This is called allosteric inhibition, which is different from how JAK [Janus kinase] inhibitors work. At therapeutic doses, deucravacitinib is intended to block TYK2 without inhibiting JAK1, JAK2, or JAK3, thus potentially avoiding adverse events associated with these inhibitors.”
This novel oral medication, which earned BMS the 2019 Thomas A. Edison Patent Award for the science underpinning its clinical development, met its primary and secondary end points in 2 phase 3 studies investigating the safety and efficacy of the drug as a treatment for moderate to severe psoriasis. POETYK PSO-1 (NCT03624127) and POETYK PSO-2 (NCT03611751) compared deucravacitinib with both placebo and apremilast (Otezla, Amgen).1,2
April Armstrong, MD, MPH, professor of dermatology and associate dean of clinical research at Keck School of Medicine at the University of Southern California in Los Angeles, presented the results from these studies at the American Academy of Dermatology Virtual Meeting Experience 2021 (AAD VMX) in April.3
“PSO-1 and PSO-2 were global, double-blinded, 52-week studies that were identical in design up to week 24,” Armstrong said. PSO-1 enrolled 666 patients and PSO-2 enrolled 1020.
Study patients who met the Psoriasis Area and Severity Index (PASI), static Physician Global Assessment (sPGA), and body surface area standards for moderate to severe plaque psoriasis were randomized using a 2:1:1 ratio into 3 possible treatment groups taking 6 mg of deucravacitinib once daily, placebo, or 30 mg of apremilast twice daily. Placebo patients were switched to deucravacitinib at week 16 in both studies. Patients taking apremilast who failed to reach PASI 50 in the PSO-1 study or PASI 75 in the PSO-2 study were switched to deucravacitinib at week 24.
“PSO-2 also included a randomized withdrawal phase at week 24,” Armstrong explained. “The results will be presented at a future date. Mean patient weight was slightly higher in PSO-2 compared [with] PSO-1, likely reflecting the difference between geographic distribution of patients.”
The study’s coprimary endpoints were the proportion of patients achieving PASI 75 or sPGA 0/1, defined as clear or almost clear responses, at Week 16 for deucravacitinib vs placebo. The baseline demographics were similar between the 2 studies and typical for trials in moderate to severe plaque psoriasis, Armstrong said. PSO-1 included sites in Asia whereas PSO-2 did not.
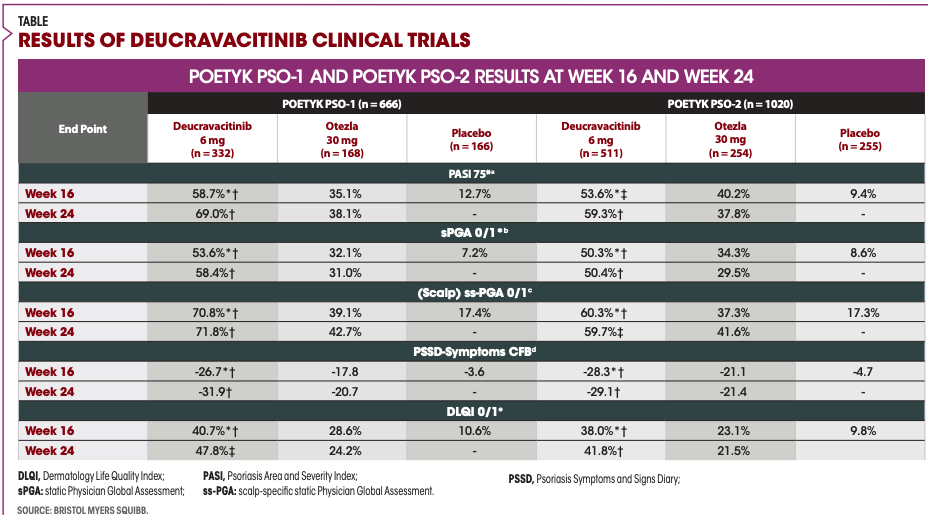
According to Armstrong, results showed that by 16 weeks, 58.7% of patients who received deucravacitinib achieved a PASI 75 response compared with 35.1% of apremilast and 12.7% placebo. This trend continued through week 24. Importantly, 82.5% of patients in PSO-1 and 81.4% in PSO-2 who achieved PASI 75 at week 16 and continued treatment maintained their response through Week 52.
For sPGA, 53.6% of patients receiving deucravacitinib in PSO-1 achieved a 0/1 response at week 16 vs 32.1% of those on apremilast and 7.2% placebo.1 These results were consistent through week 24 and comparable with the PSO-2 trial.
More than 60% of patients had moderate to severe plaque psoriasis affecting the scalp at baseline. The breakdown of patients who met scalp-specific sPGA 0/1 responses by week 16 in PSO-1 was as follows: 70.8% of patients receiving deucravacitinib vs 39.1% with apremilast and 17.4% with placebo. These outcomes were similar to those observed in PSO-2, according to Armstrong.
“Improvements were seen in base in PSSD [Psoriasis Symptoms and Signs Diary] symptoms, which include itching, pain, stinging, and skin tightness,” Armstrong said.
Approximately 95% of patients also had Dermatology Life Quality Index (DLQI) scores of greater than or equal to 2 at baseline. More than 40% of patients taking deucravacitinib compared with 28.6% of patients taking apremilast and 10.6% of patients taking placebo achieved DLQI 0/1 response at week 16 in PSO-1.1 Deucravacitinib’s effectiveness was also greater than apremilast’s effectiveness through week 24.
Deucravacitinib, apremilast, and placebo had a similar total number of adverse events (AEs) and serious adverse events (SAEs). These numbers include both the PSO-1 and the PSO-2 trials. Deucravacitinib had 2.4% of AEs that led to discontinuation. This was less than 3.8% with placebo and 5.2% with apremilast.
The most common AEs across treatment groups were nasopharyngitis, upper respiratory tract infection, headache, diarrhea, and nausea. One death occurred in each of the treatment groups, but none was attributed to the study.
“Due to the randomizations at week 16 and week 24 from apremilast, the total exposure to deucravacitinib is nearly 1000 patient years, with less than 250 patient years for placebo and apremilast,” Armstrong said. “The safety profile for weeks 0 to 52 is similar to those observed from week 0 to 16 with no new safety findings.”
Deucravacitinib had a low incidence rate of approximately 2% for patients who experienced folliculitis and acne and led 1 patient to discontinue use. The exposure-adjusted incidence rate (EAIR) for AEs of interest showed malignancies and serious infections had similar rates across treatment groups in both studies. None of the patients who had a serious infection on deucravacitinib discontinued the study.
There were 4 arterial thrombotic events reported in the deucravacitinib group, 1 of which was considered serious. There was 1 SAE adjudicated as a venous thromboembolism at week 48. This patient was hospitalized, underwent an aortic repair, recovered, and restarted treatment with deucravacitinib for the long-term study.
The study also looked at 4 laboratory parameters that can be affected within the first 4 months of treatment with JAK inhibitors. “Importantly, no clinically meaningful changes were observed in total cholesterol, creatine phosphokinase, neutrophils, or platelets with deucravacitinib at 16 weeks,” Armstrong said. “Similar results were observed in 52 weeks in these and other parameters.”
In both studies, deucravacitinib demonstrated superiority compared with placebo and apremilast in both coprimary end points at week 16; therapeutic effect was maintained through week 52. Also, deucravacitinib had a consistent safety profile between both studies and was well tolerated.
“Based on these findings, deucravacitinib has the potential to become an efficacious, well-tolerated treatment of choice for patients with moderate to severe plaque psoriasis,” Armstrong said. “It also has significant potential to be broadly applicable to a range of immune-mediated diseases such as psoriatic arthritis, ulcerative colitis, Crohn disease, and lupus.”
Disclosures:
April Armstrong, MD, MPH, receives grants and personal fees from AbbVie, Bristol Myers Squibb, Eli Lilly, Janssen Pharmaceuticals, Leo Pharma, and Novartis; personal fees from Boehringer Ingelheim/Parexel, Celgene, Dermavant, Genentech, GlaxoSmithKline, Menlo Therapeutics, Merck, Modernizing Medicine, Ortho Dermatologics, Pfizer, Regeneron, Sanofi Genzyme, Science 37, Sun Pharmaceuticals, and Bausch Health; and grants from Dermira, Kyowa Kirin, and UC outside the submitted work.
References:
1. Effectiveness and safety of BMS-986165 compared to placebo and active comparator in participants with psoriasis (POETYK PSO-1). ClinicalTrials.gov. Updated February 5, 2021. Accessed May 14, 2021. https://clinicaltrials.gov/ct2/show/NCT03624127?term=POETYK+PSO-1+%28NCT03624127%29&draw=2&rank=1
2. An investigational study to evaluate experimental medication BMS-986165 compared to placebo and a currently available treatment in participants with moderate-to-severe plaque psoriasis (POETYK-PSO-2). ClinicalTrials.gov. Updated August 28, 2020. Accessed May 14, 2021. https://clinicaltrials.gov/ct2/show/NCT03611751?term=POETYK+PSO-2+NCT03611751&draw=2&rank=1
3. Armstrong A. Efficacy and safety of deucravacitinib, an oral, selective tyrosine kinase 2 (TYK2) inhibitor, compared with placebo and apremilast in moderate to severe plaque psoriasis: results from the phase 3 POETYK PSO-1 POETYK PSO-2 studies. Presented at: American Academy of Dermatology Virtual Meeting Experience 2021; April 23-25,2020; virtual.

Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.