- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Publication
Article
Dermatology Times
FDA Accepts NDA, Sets Action Date for Apremilast
Author(s):
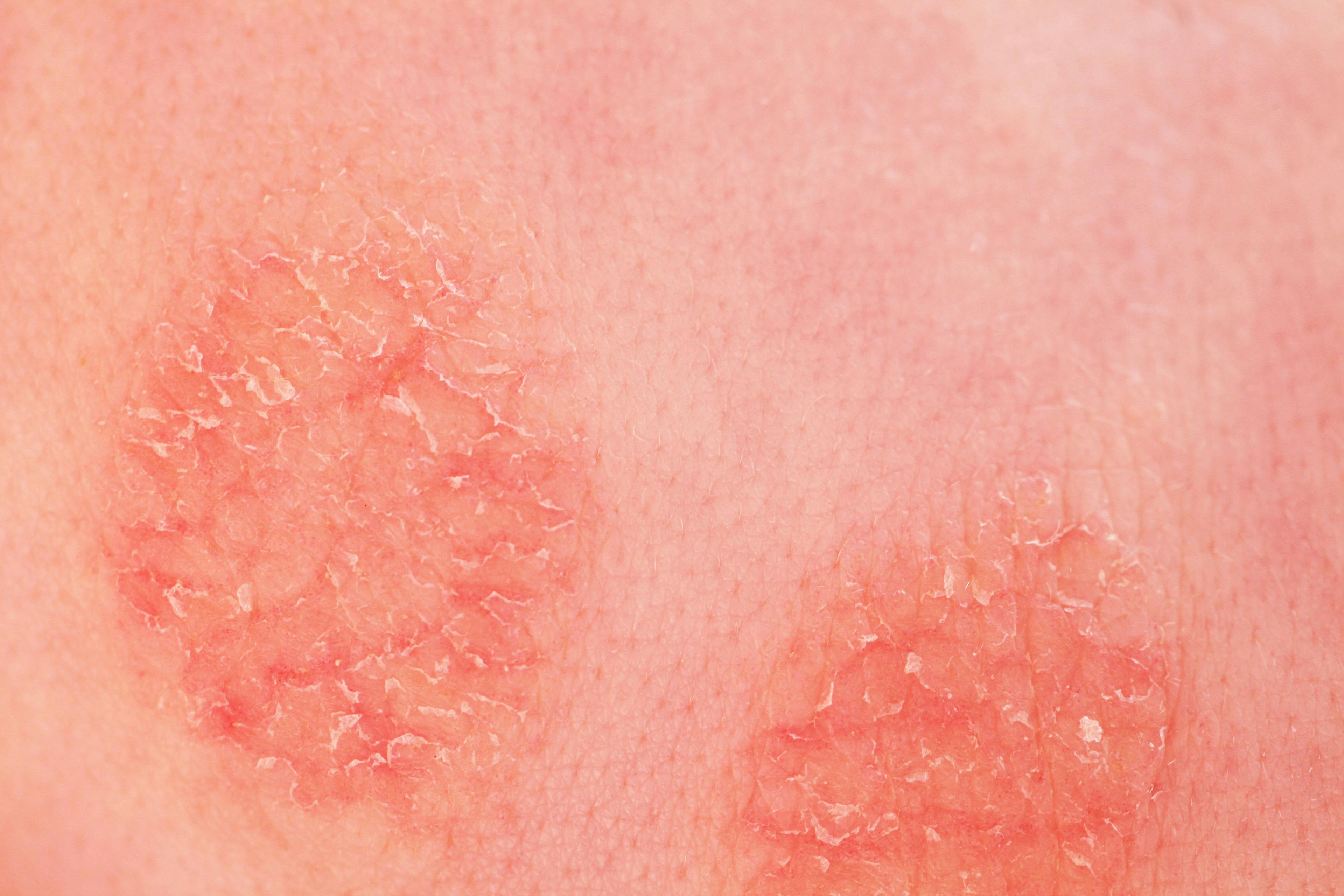
After data from a phase 3 study, FDA opens potential for apremilast to be the first and only approved oral therapy for mild to moderate plaque psoriasis.
The FDA has accepted the supplemental New Drug Application (sNDA) and set a Prescription Drug User Fee Act (PDUFA) date for apremilast (Otezla, Amgen). The FDA will decide on whether to approve the drug or not for the treatment = of adults with mild to severe plaque psoriasis on December 19, 2021.1
Currently, the drug is approved for the treatment of moderate to severe plaque psoriasis in adult patients who are candidates for phototherapy or systemic therapy, adult patients with active psoriatic arthritis and adult patients with oral ulcers associated with Behçet's Disease.1
"Otezla has been prescribed to hundreds of thousands of patients with moderate to severe plaque psoriasis. Based on the positive phase 3 ADVANCE data, we believe it could play an important role in addressing the unmet need for adults affected by mild-to-moderate plaque psoriasis who have had challenges managing their disease with existing topical therapies alone," said David M. Reese, MD, executive vice president of Research and Development at Amgen. "The FDA's acceptance of this sNDA for Otezla is a significant milestone toward achieving our goal of potentially providing the first and only oral treatment option for the underserved mild-to-moderate patient population."
As previously reported by Dermatology Times, the sNDA acceptance is supported by data from the phase 3 ADVANCE study (NCT03721172). ADVANCE was a double-blind, multicenter, placebo-controlled trial that examined apremilast in 595 adult subjects with mild-to-moderate plaque psoriasis who were given 30 mg of apremilast or placebo twice daily for 16 weeks then followed by an open-label extension study that extended to Week 32.
Results demonstrated a positive outcome with apremilast achieving its primary endpoint of a statically significant improvement in Static Physicians Global Assessment (sPGA) response at Week 16 versus placebo. The secondary endpoints in the study were also met with a minimum of 75% improvement from baseline in percent of affected body surface area (BSA), an alteration in Psoriasis Area and Severity Index (PASI) score from baseline and a change in BSA score from baseline.2
Safety was also evaluated in the study with the safety profile mimicking those in previous studies. The most common treatment-related adverse events (≥5%) include nausea, headache, nasopharyngitis, upper respiratory tract infection and diarrhea. The is consistent with previously reposted data from clinical trial programs.
Reference:
1. Amgen. FDA accepts Amgen’s supplemental new drug application for Otezla® (Apremilast) for adults with mild-to-moderate plaque psoriasis. Accessed May 6, 2021. https://www.prnewswire.com/news-releases/fda-accepts-amgens-supplemental-new-drug-application-for-otezla-apremilast-for-adults-with-mild-to-moderate-plaque-psoriasis-301283931.html
2. Amgen Announces Positive TopLine Results From Otezla apremilast Phase 3 ADVANCE Study In MildToModerate Plaque Psoriasis. Amgen, Inc. https://www.amgen.com/media/news-releases/2020/05/amgen-announces-positive-topline-results-from-otezla-apremilast-phase-3-advance-study-in-mildtomoderate-plaque-psoriasis/. Published May 6, 2020.

Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.