- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Publication
Article
Dermatology Times
First approved biologic in dermatology to carry suffix
Author(s):
Biologic tildrakizumab-asmn approved with suffix as differentiator from future biosimilar versions.
Biologic tildrakizumab-asmn approved with suffix as differentiator from future biosimilar versions. (ArtCookStudio - stock.adobe.com)
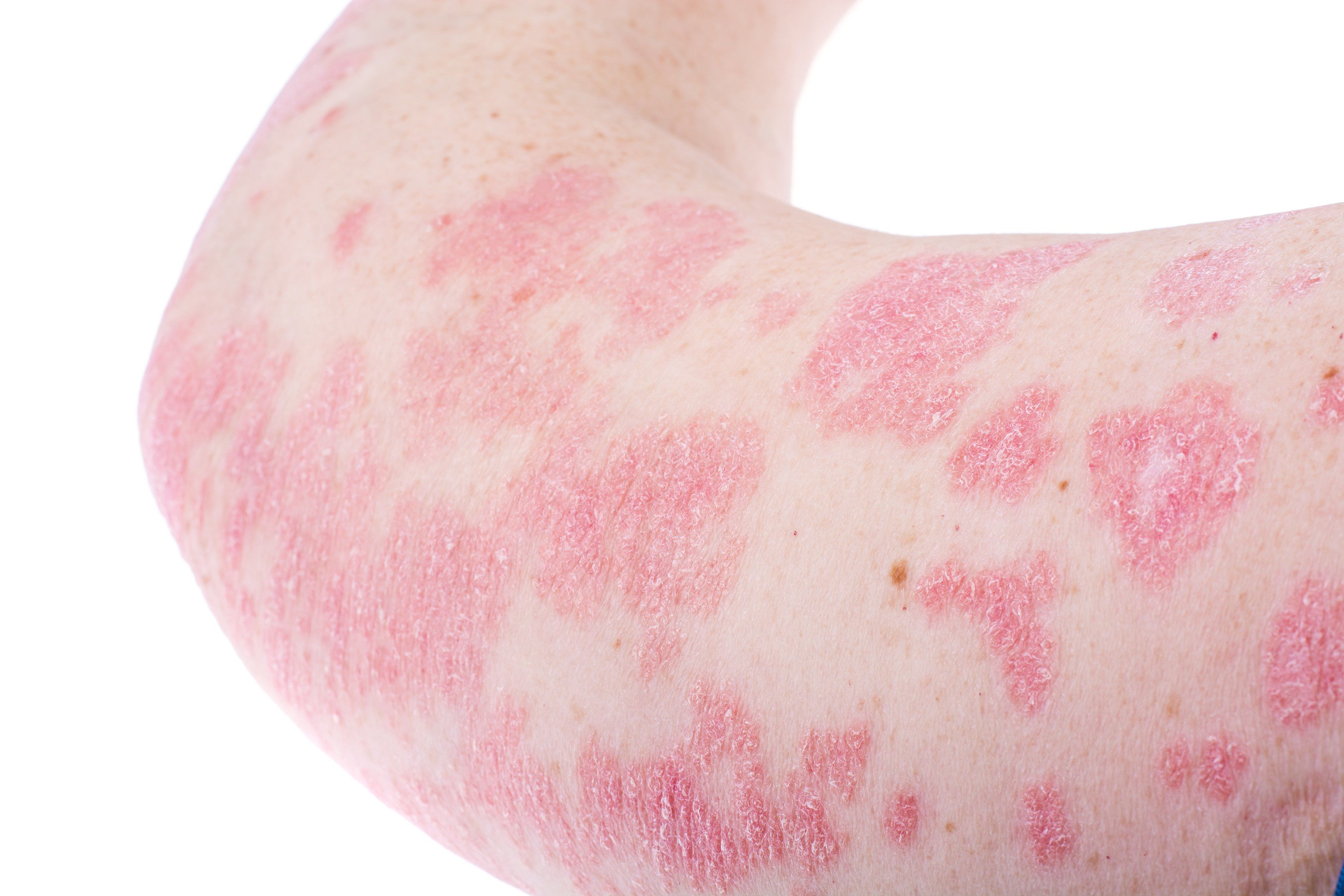
Tildrakizumab-asmn (Ilumya, Sun Pharmaceuticals), the interleukin (IL)-23-inhibitor biologic approved for treating moderate-to-severe psoriasis, is the first originator biologic in dermatology to adopt the 2017 FDA-mandated “asmn” suffix. It behooves dermatologists to understand this naming convention as new biologics and biosimilars become available.
This four-letter nomenclature has been used to identify biosimilars in dermatology since 2016.
The U.S. Food and Drug Administration introduced guidance in January 2017 recommending that all biological products include an FDA-designated suffix to help patients and physicians accurately discern between biologic products, related biologic products and biosimilars; facilitate pharmacovigilance when tracking biologic ordering, prescribing, and dispensing; and to minimize inadvertent substitution of products that have not been determined to be interchangeable.1
Last November, the FDA added fourletter meaningless suffixes to two newly approved biologics outside of dermatology: emicizumab-kxwh (Hemlibra, Roche) for hemophilia and vestronidase alfa-vjbk (Mepsevii, Ultragenyx Pharmaceutical) for the rare inherited condition Sly syndrome.
Pharmaceutical companies can propose a maximum of 10 suffixes for their product, as long as they are not perceived as confusing. The suffixes are a combination of four letters that carry no meaning, and the FDA makes the final selection.
In a June 2018 article in the American Journal of Clinical Dermatology, first author Eric Yang, M.D., of the department of dermatology at the University of California, San Francisco, and colleagues note that “adoption of this nomenclature by this newly approved drug demonstrates an anticipated increased role of biosimilars in dermatology.”2
Simon Lowry, M.D., head of Medical Affairs and chief medical officer for Sun Pharma, notes, “The assigned meaningless suffix ‘asmn’ is used to differentiate our originator biologic from future biosimilar versions of tildrakizumab.”
Tildrakizumab-asmn was approved by the FDA in March 2018 for the treatment of adults living with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy.
“There is a need for additional treatment options for people living with the persistent nature of moderate-to-severe plaque psoriasis,” Dr. Lowry tells Dermatology Times.
The approval of tildrakizumab-asmn was supported by data from two multicenter, randomized, double-blind, placebo-controlled trials (reSURFACE 1 and reSURFACE 2) in which 926 patients with moderate-to-severe chronic plaque psoriasis were treated with tildrakizumabasmn, etanercept (reSURFACE 1) or placebo. In reSURFACE 1, 74% of patients treated with tildrakizumab-asmn 100 mg achieved 75% skin clearance at week 28 after three doses. In addition, 84% of patients who continued receiving tildrakizumab-asmn 100 mg maintained PASI 75 at week 64 compared to 22% of patients who were re-randomized to placebo.3
Further research from a long-term pooled analysis found that 9 out of 10 patients on tildrakizumab-asmn who achieved PASI 75 at week 28 maintained their skin clearance after three years of treatment.4
“Over the three-year period, the drug was well-tolerated with a low rate of adverse events of interest,” Dr. Lowry says.
The three most common adverse reactions associated with 100 mg dosing are upper respiratory infections, injection site reactions and diarrhea.
Dr. Lowry says the IL-23 genetic pathway is present in numerous autoimmune diseases, including psoriatic arthritis and ankylosing spondyloarthritis, for which the company is currently conducting phase 2 studies with tildrakizumabasmn.
Disclosure:
Simon Lowry is an employee of Sun Pharma.
Reference
1 Nonproprietary Naming of Biological Products. U.S. Food and Drug Administration. January 2017. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM459987.pdf.
2 Yang E, Beck K, Liao W. “Tildrakizumab-asmn: What’s in a Name?” American Journal of Clinical Dermatology, June 2018, 19(3):291-292. DOI: 10.1007/ s40257-018-0357-6.
3 Sun Pharma Announces U.S. FDA Approval of ILUMYA™ (tildrakizumab-asmn) for the Treatment of Moderate-toSevere Plaque Psoriasis. Sun Pharma. March 21, 2018. https://www.prnewswire.com/news-releases/sunpharma-announces-us-fda-approval-of-ilumya-tildrakizumab-asmn-for-the-treatment-of-moderate-to-severeplaque-psoriasis-300617454.html.
4Thaçi D, et al. Long-term efficacy and safety of tildrakizumab in patients with moderate-to-severe psoriasis who were responders at week 28: pooled analysis through 3 years. Presented at EADV, September 2018.

Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.