- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Publication
Article
Dermatology Times
Harnessing the Immune System to Fight Basal Cell Carcinoma: What are the Risks?
Author(s):
In basal cell carcinoma, the most common type of skin cancer accounting for 80% of all non-melanoma skin cancers, one of the leading options for cases that fall into this category is targeted treatment with Hedgehog pathway inhibitors.
Resection or surgical removal is one of the primary methods for managing skin cancers, but it’s not always an option. In basal cell carcinoma, the most common type of skin cancer accounting for 80% of all non-melanoma skin cancers, one of the leading options for cases that fall into this category is targeted treatment with Hedgehog pathway inhibitors. However, a new study questions the impact these medications could have on the overall health and function of the systemic immune system.
The study, published in 2022 in Frontiers in Medicine examines the immune response used in targeted basal cell carcinoma treatments, and the effect these medications could have on overall immune health, as well as any uses in treating other neoplasms.
The Hedgehog signaling pathway is where basal cell carcinoma develops at the molecular level. Medications that target this pathway have been approved to treat basal cell carcinoma in local advanced stages—particularly when surgical management isn’t an option.
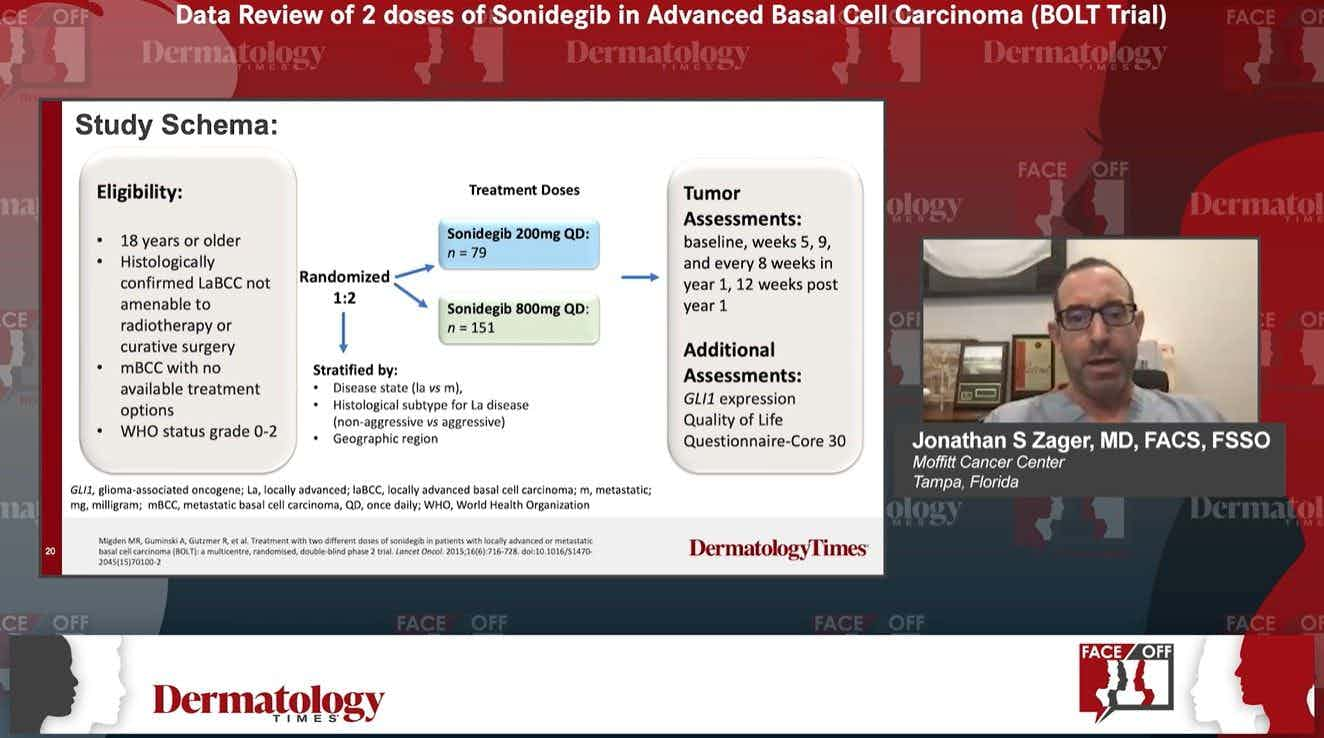
The results of the medications that fall into the Hedgehog pathway inhibitor category are good, in general, according to the study. There are two main options for specific medications in this category--vismodegib and sonidegib. An immunocheckpoint inhibitor called cemiplimab is also an option is cases where systemic therapy is preferred over surgical or radiation treatments.
Sporadic and germline mutations are common in the pathogenesis of basal cell carcinoma, but immunotherapy has opened new doors for treatment. Regulatory T-cells create an immunosuppressive effect, and are typically found in large concentrations in tissues affected by basal cell carcinoma, according to research. Other elements that can contribute to the formation or metastasis of BCC include UV exposure, genetics, and family history.
Vismodegib, approved by the U.S. Food and Drug Administration in 2012 under the trade name Erivedge has a strong inhibiting effect on the Hedgehog pathway, and was the first medication of its kind approved for treating basal cell carcinoma. The second option is sonidegib, approved in 2015 under the trade name Odomzo. This medication works by preventing signaling in the Hedgehog pathway.
Both of these medications have demonstrated strong responses—roughly 50% to 70% —in patients with local advanced basal cell carcinoma. However, the study authors note that there is little information available on how these medications impact the other functions of the systemic immune system in people who are being treated for BCC. In animal models, a number of reductions were observed in several types of immune cells, but the impact of these reductions appeared limited to reduced local inflammation. More research is needed to determine the overall systemic immune response of medications like vismodegib and sonidegib, the report notes.
Reference
- Gambini D, et al. Basal cell carcinoma and Hedgehog pathway inhbitors: Focus on immune response. Front Med. June 2022. doi:10.3389/fmed.2022.893063.

Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.