- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Publication
Article
Dermatology Times
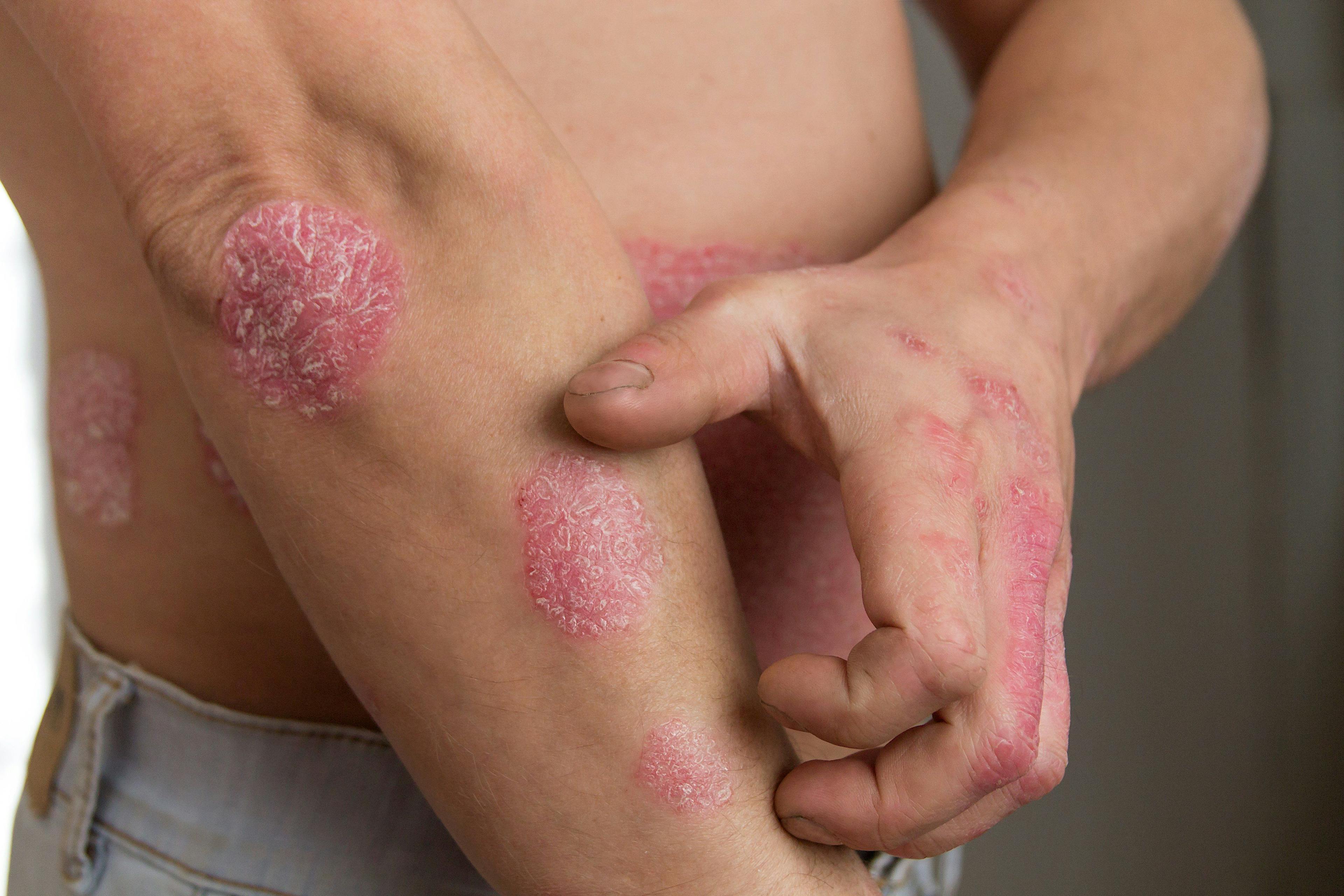
New AAD-NPF Psoriasis Guidelines Mark Changes
Author(s):
New AAD-NPF guidelines focus on biologics, topical therapy, systemic non-biological therapies, management of pediatric patients, use of phototherapy and comorbidities.
The first update in more than a decade, the most recent version of the American Academy of Dermatology (AAD)-National Psoriasis Foundation (NPF) guidelines identify some substantive changes in the management and treatment of psoriasis.
The newly released guidelines cover six topics: biologics, topical therapy, systemic non-biological therapies, management of pediatric patients, use of phototherapy, and comorbidities. Abby S. Van Voorhees, MD, highlighted these updates at the ODAC 2021 Pre-Conference Sneak Peek Inflammatory Diseases Virtual Symposium held in December.1
One key change is the timeline for treatment success or failure. She used a case study of a 42-year-old male patient with psoriasis and inflammatory bowel disease who experienced a worsening flare of psoriasis despite being on adalimumab for 32 weeks to illustrate the failure in treatment caused by the biologic not working.
“A primary failure of a biologic medication is when a disease doesn’t respond to the initial course of treatment within the expected timeline,” she says. A secondary failure is when the disease responds initially, but skin clearance is lost over time. Practitioners need to understand how long to give a treatment to work and how to define what’s too short or too long, according to Van Voorhees, professor and chair of dermatology at Eastern Virginia Medical School, Norfolk, Va.
Medications vary for peak efficacy. For example, she says adalimumab has a peak efficiency at week 16. While the patient in the case study did well initially, the drug lost its efficacy and experienced secondary biologic failure. Practitioners should be aware of each drug’s efficacy timelines. For tumor necrosis factor (TNF) inhibitors, peak efficacy for etanercept is week 12, for infliximab week 10, and for adalimumab week 16. For interleukin (IL)-12/23 ustekinumab, peak efficacy is week 12; for IL-23 guselkumab it is week 16; for tildrakizumab and risakizumab week 12. Lastly for IL-17, peak efficacy occurs for secukinumab, ixekizumab, and brodalumab at week 12.
Van Voorhees also discussed the new onset of psoriasis or the paradoxical worsening of psoriasis in those treated with TNF inhibitors, which is commonly seen in patients with inflammatory bowel disease (IBD). She says it can mimic psoriasis and frequently involves the palms and soles. Treatments to relieve symptoms include class 1 steroids, an alternative biologic within a class of medication, and a change of biologic agent to an alternate class.
An increased risk of some types of cancer is inherent to psoriasis. The most common malignancies among psoriasis patients are lymphohematopoietic (with a standard incidence rate of 15.1), pancreatic, oropharynx, and larynx, in addition to nonmelanoma skin cancer, especially squamous cell carcinoma. However, there is no increased risk of melanoma or non-Hodgkin lymphoma, she adds.
The new guidelines divide surgical procedures into low risk, moderate risk, and high risk. Van Voorhees says that procedures that interrupt the respiratory passages or GI or urinary tract can be considered high risk. However, procedures such as orthopedic surgery or dermatologic surgery would identify as low risk.
In a low-risk situation, patients can continue on their drugs without interruption, she says. That may not be the case if the patient requires a significant respiratory procedure, which requires discontinuation of biologics. The risk of a surgical procedure depends on the procedure under consideration, according to Van Voorhees.
Drug treatment guidelines reflect key changes as well. “The new recommendations for liver toxicity associated with methotrexate require considering performing serologic studies and the liver stiffness assessment studies,” she says.
“We have begun to understand that the liver biopsy itself carries its own morbidity and risk, therefore, the new guidelines emphasize that liver biopsies are not needed.
Methotrexate should not be given to psoriasis patients who are pregnant, nursing, have a history of alcoholism or a history of liver disease, immunodeficiency syndromes, bone marrow hypoplasia, leukopenia, thrombocytopenia, or significant anemia, or those who are sensitive to the drug.
Relative contraindications include abnormalities in renal or liver function or active infection. Risk factors for hepatotoxicity include alcohol consumption, persistent abnormal liver function tests, chronic hepatitis B or C, a family history of inheritable liver disease, diabetes, obesity, exposure to hepatotoxic chemicals or drugs, and hyperlipidemia.
Van Voorhees notes that psoriasis is connected to many comorbidities, including metabolic syndromes such as obesity, hypertension, dyslipidemia, and diabetes. The risk of sleep apnea is increased, she adds. There is also an increased risk of uveitis, depression, chronic obstructive pulmonary disease (COPD), kidney disease, cardiovascular disease, sexual dysfunction, as well as cigarette smoking and alcohol abuse. Van Voorhees emphasizes the importance of screening psoriasis patients for comorbidities so patients can receive proper treatment.
Disclosures: Dr. Van Voorhees reports disclosures related to Celgene, AbbVie, Lilly, Novartis, and UCB.
Reference:
1. Van Voorhees, AS. Psoriasis management guidelines: what you need to know. Presented at: 2021 ODAC Pre-Conference Sneak Peek Inflammatory Diseases Virtual Symposium; December 3, 2020.

Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.