- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Publication
Article
Dermatology Times
New Treatments Aim to Increase Survival Rate
Author(s):
Cancer staging systems for squamous cell carcinoma have helped improve dermatologists’ ability to accurately stage the cancer, according to one expert.
Cutaneous squamous cell carcinoma (cSCC) appears to be far surpassing melanoma as a cause of skin cancer– related death. Each year, more than 15,000 individuals in the United States die of cSCC, which is more than twice the number of deaths related to melanoma.1
This concerning statistic has captured the attention of the industry and investigators, who are developing new ways to assess squamous cell carcinoma risk and better treat the disease.
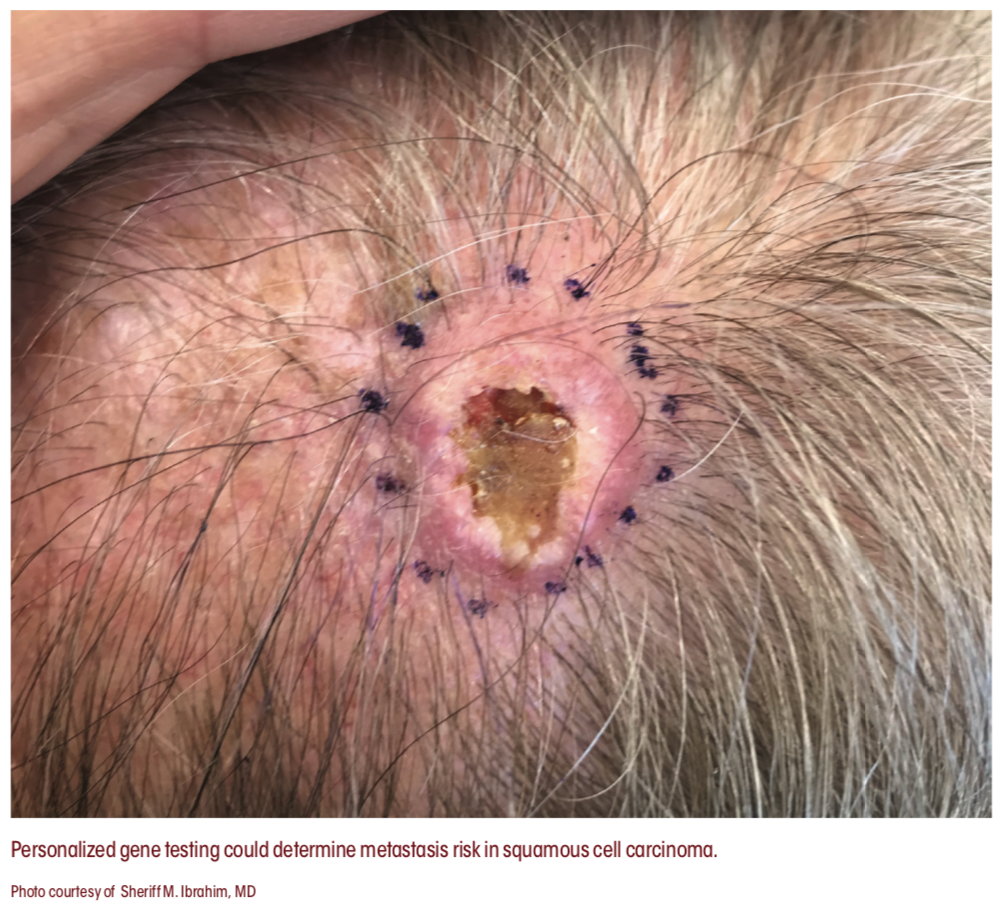
INDIVIDUALIZED RISK ASSESSMENT BY GENE EXPRESSION PROFILING
Cancer staging systems for squamous cell carcinoma, including those of the American Joint Committee on Cancer and Brigham and Women’s Hospital, have helped improve dermatologists’ ability to accurately stage the cancer, according to Sherrif F. Ibrahim, MD, PhD, an associate professor of dermatology at University of Rochester Medical Center in New York and a Skin Cancer Foundation spokesperson. “When we stage cancers, however, it doesn’t really help the individual. It tells us that if you take every- body with a given stage [of] squamous cell carcinoma, they have a certain chance of having a poor outcome,” Ibrahim said.
A new type of testing might be more useful to individual patients, according to Ibrahim. In September 2020, the (FDA) approved a novel personalized gene expression profile test indicated for use in squamous cell carcinoma, DecisionDx-SCC (Castle Biosciences).
“Using this test, we can actually study individual genes in a given patient’s squamous cell carcinoma and determine their risk for metastasis,” Ibrahim said. “What we are hoping for is... to approach staging on an individualized level, not just in an individual patient, but even for an individual cancer on a patient who has multiple cancers. And we can determine which of these patients might need closer monitoring, closer follow-up, more imaging, and perhaps additional treatments down the road.”
Castle Biosciences has a similar approved test for melanoma. “Individualized risk assessment by gene expression profiling is an exciting development, but of course more work needs to be done to determine how these tests will become incorporated in our day-to-day management of these diseases,” Ibrahim said.
Skin cancer care is moving in the direction of more accurate risk assessment coupled with more personalized treatment. This will be especially valuable in the setting of cSCC, Ibrahim said: “We do not have good guidelines on how often to image patients, what type of imaging to use, and what type of adjuvant treatment would most benefit high-risk squamous cell carcinoma patients. But with this individualized genetic testing, we might be able to really start probing those questions to develop more structured guidelines for the management of more advanced cases, of which we are seeing a lot more.”
NEW AND EMERGING THERAPIES FOR CSCC AND BASAL CELL CANCER
The FDA has approved 2 checkpoint inhibitors, cemiplimab (Libtayo; Regeneron Pharmaceuticals) and pembrolizumab (Keytruda; Merck), for locally advanced or metastatic squamous cell carcinoma. Pembrolizumab received the OK for treatment of recurrent or metastatic cSCC in June 2020.1 The FDA approved cemiplimab in September 2018 for patients with metastatic or locally advanced cSCC who are not candidates for curative surgery or curative radiation.2
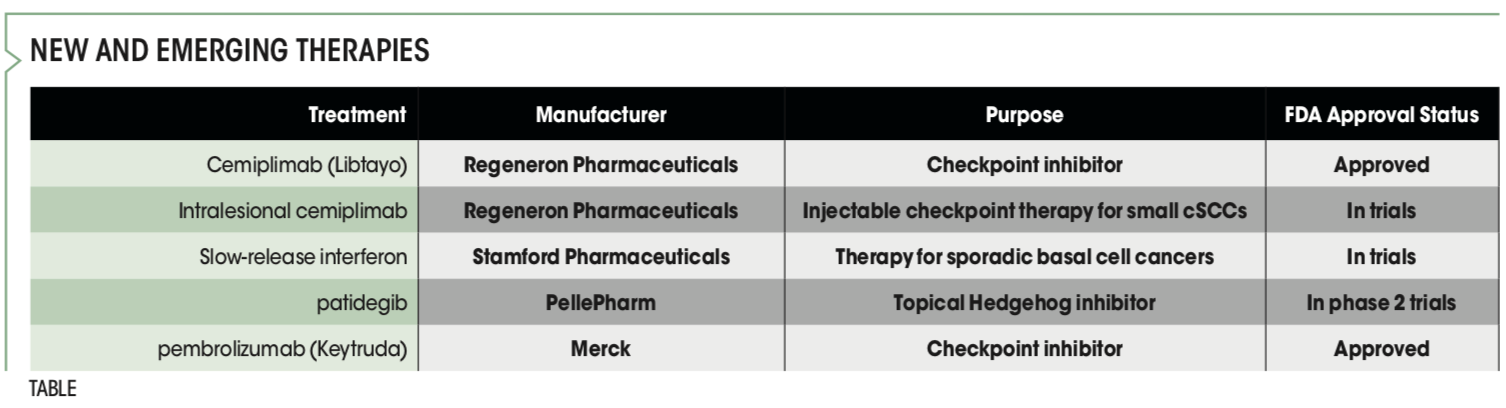
“A lot of people do not realize that there are these new and other emerging therapies,” Ibrahim said. “We have seen great advances for locally advanced nonmelanoma skin cancer, including the Hedgehog inhibitors vismodegib and sonidegib for basal cell carcinoma.”
Treatment of small sporadic nonmelanoma skin cancers also is advancing, including many injectable and topical agents, according to Ibrahim. “For instance, we have a trial under way with Regeneron that is looking at intralesional immune checkpoint inhibitor treatment. It includes injectable therapy for small, 1- to 2-cm squamous cell carcinomas. The hope is that we will have excellent results without the [adverse] effect profile of delivering these drugs systemically,” he said.
Ibrahim is among the researchers studying an investigational virally mediated interferon. Interferon works well for basal cell cancers when injected, but the required 4 or 5 painful shots a week for several weeks is prohibitive. Stamford Pharmaceuticals’ new agent, an injectable slow-release interferon, calls for just 1 injection per week for 3 weeks. “We expect to achieve over 90% clearance for sporadic basal cell cancers using this therapy,” Ibrahim said.
A topical Hedgehog inhibitor, patidegib, has moved further through the pipeline and is in phase 2 trials. When given systemically, Hedgehog inhibitors have a high adverse effect (AE) profile. The hope is that this topical agent from PellePharm may help patients with or at risk for basal cell carcinoma with fewer AEs.
Intralesional treatments for run-of-the-mill small skin cancers will provide a much-needed nonsurgical treatment option, Ibrahim said. “It is an exciting and rapidly changing time in the field of skin cancer,” he said. “With many new advances in understanding the genetic underpinnings of disease and better nonsurgical options to treat all severities of tumors, we hope to bring better outcomes to our patients.”
Disclosure:
Ibrahim is an adviser, a speaker, and an investigator for Castle Biosciences, Genentech, and Regeneron; an adviser and a speaker for SUN Pharmaceuticals and an advisor and investigator for Stamford Pharmaceuticals.
References:
1. Skin cancer facts & statistics. Updated April 2020. Accessed Janu-
ary 8, 2021. The Skin Cancer Foundation. https://www.skincancer.org/ skin-cancer-information/skin-cancer-facts/
2. FDA approves pembrolizumab for cutaneous squamous cell carcinoma. US Food and Drug Administration. June 24, 2020. Accessed Janu- ary 8, 2021. https://www.fda.gov/drugs/drug-approvals-and-databases/ fda-approves-pembrolizumab-cutaneous-squamous-cell-carcinoma
3. FDA approves cemiplimab-rwlc for metastatic or locally advanced cutaneous squamous cell carcinoma. US Food and Drug Administration. January 18, 2019. Accessed January 8, 2021. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-cemiplimab-rwlc-metastatic-or-locally-advanced-cutaneous-squamous-cell-carcinoma

Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.