- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Publication
Article
Dermatology Times
AK Overview: Current Guidelines and Emerging Therapies
Author(s):
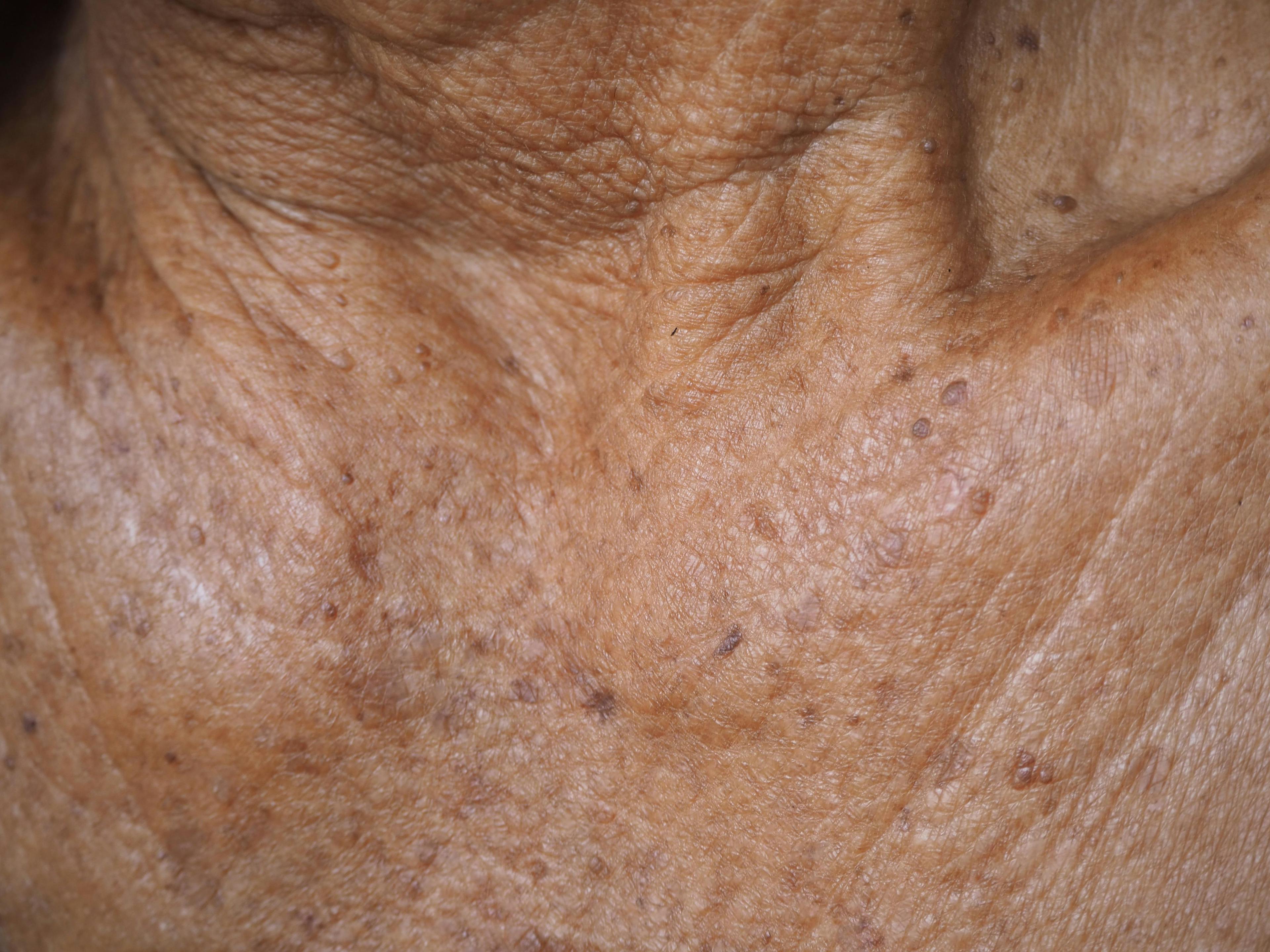
A review of the American Academy of Dermatology guidelines and current treatment improvements for actinic keratosis were discussed during a presentation at Maui Derm NP+PA Fall 2021.
A variety of factors are altering the treatment landscape for actinic keratosis (AK), according to a presenter at Maui Derm NP + PA Fall 2021 who discussed the driving factors recently published American Academy of Dermatology (AAD) guidelines, as well as innovations in therapies and approaches.
In a session at the conference on cutaneous oncology, George Martin, MD, program chairman of Maui Derm NP+PA Fall 2021 and a dermatologist at Dr George Martin Dermatology Associates in Kihei, Hawaii, outlined what’s ahead for AK solutions. Ted Rosen, MD, a professor of dermatology at Baylor College of Medicine in Houston, Texas, collaborated on the presentation materials.1
GUIDELINES REQUIRE EXPANSION
Martin began his presentation discussing his concerns regarding the AAD’s guidelines of care for managing AK.2 “The guidelines were limited by a database search that was outdated by 2 years, not mentioning actinic cheilitis, and not providing guidance on immunocompromised patients,” he said.
Martin said, prescribing topical creams, gels, and solutions for patients with AK is common practice. Based on current literature, the guideline authors strongly supported treatment with either 5-fluorouracil (5-FU) or imiquimod.
However, Martin commented on the guidelines’ lack of discussion regarding new options such as nicotinamide-specific methods of cryosurgery, as well as short-incubation and simultaneous-incubation illumination photodynamic therapy (PDT). The guidelines also do not give specific recommendations on tirbanibulin (Klysiri; Athenex and Almirall) or discuss the reason for withdrawing ingenol mebutate from the market, he said.
He sees promise for tirbanibulin 1% topical cream, which the FDA approved in 2020 for the treatment of AK.3 The topical first-in-class microtubule inhibitor plays a role in cell structure, transport, and division.
Martin presented data from a phase 3 study (NCT03285477) showing that 44% of patients treated with tirbanibulin 1% achieved 100% clearance at day 57 compared with 5% of patients who received placebo. Broken down further, the findings suggest that this treatment is more effective on the face (50%-61%) than the scalp (30%-40%). This rate was significantly higher for greater than 75% clearance: 76% to 80% on the face vs 52% to 69% on the scalp for patients treated with tirbanibulin 1% cream.
Besides being effective, Martin said, tirbanibulin 1% cream was well tolerated, with just 1% of patients treated experiencing vesiculation/pustulation and none reporting erosion/ulceration. Skin irritability peaked at day 8 and had a recurrence rate at 1 year of 73%, with a true recurrence rate of 47%. This may be due to new lesions not identified at baseline or true recurrence, Martin explained. In either case, he said, a patient would likely have just 1 or 2 AK lesions after treatment.
Despite the positive outcomes, questions remain, according to Martin: The study is limited by the area of the body on which tirbanibulin 1% was tested. He advised against pre- scribing the cream for patients who are pregnant or planning to become pregnant, with the same precautions spelled out in 5-FU guidance. Cost could also be an impediment for patients, he added.
NEW APPROACH REDUCES PDT PAIN LEVELS
Although effective, PDT is often painful. New research findings show that a simultaneous application of 20% 5–aminolevulinic acid (ALA) solution and illumination using blue light can lessen a patient’s pain, Martin said.
He cited a 2020 study that investigated a regimen to help minimize pain during blue light PDT for AK.4 The study had a “split face” approach, with 1 side (A) treated with 30-, 45-, and 60-minute ALA incubation along with blue light, whereas the other side (B) followed the standard of 60 minutes of ALA incubation followed by 1000 seconds of blue light.
The study authors reported that the efficacy was the same. However, Martin noted that the pain score for the side A was much lower than for side B.
Martin’s best practices for treating patients with PDT are the following:
- Apply the ALA.
- Immediately turn on the blue light.
- Illuminate for 30 minutes.
He noted that data for ALA-PDT treatment for upper extremity suggest that targeted treatment of grade 4 to 15 AK at baseline and week 8, which had a 3-hour incubation period, was effective. At week 8, 67.3% of AKs cleared; at week 12, 80.6% cleared with ALA-PDT treatment.
With these new changes, PDT-ALA can be not only effective but also less painful for treating patients, which is his goal, Martin said.
Disclosures:
George Martin is on the scientific advisory board for Bristol Meyers Squibb, DUSA/SUN, AbbVie, Ortho/Bausch Health Galderma, Pfizer, Leo Pharma, Celgene, Janssen, Horizon, and UCB. He is a consultant for DUSA/SUN, Bristol Meyers Squibb, Ortho/Bausch, Pfizer, Almirall, Celgene, Lilly, Athenex, Biofrontera, UCB, and Leo Pharma.
References:
1. Martin G. Cutaneous Oncology. Session presented at: Maui Derm NP+PA Fall 2021 conference Program; September 30, 2021; Accessed September 30, 202. Asheville, North Carolina
2. Eisen DB, Asgari MM, Bennett DD, et al. Guidelines of care for the management of actinic keratosis. J Am Acad Dermatol. 2021;85(4):e209-e233.
3. FDA approves topical for actinic keratosis. Dermatology Times. Published December 16, 2020. Accessed September 30, 2021. https://www.dermatologytimes.com/view/fda-approves-topical-for-actinic-keratosis
4. Kaw U, Ilyas M, Bullock T, et al. A regimen to minimize pain during blue light photodynamic therapy of actinic keratoses: Bilaterally controlled, randomized trial of simultaneous versus conventional illumination. J Am Acad Dermatol. 2020;82(4):862-868. doi:10.1016/j.jaad.2019.09.010

Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.