- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Publication
Article
Dermatology Times
Dupilumab Efficacious in Pediatric Patients as Young as 6 Months
Author(s):
The drug was both safe and effective in patients ages 6 months to 5 years.
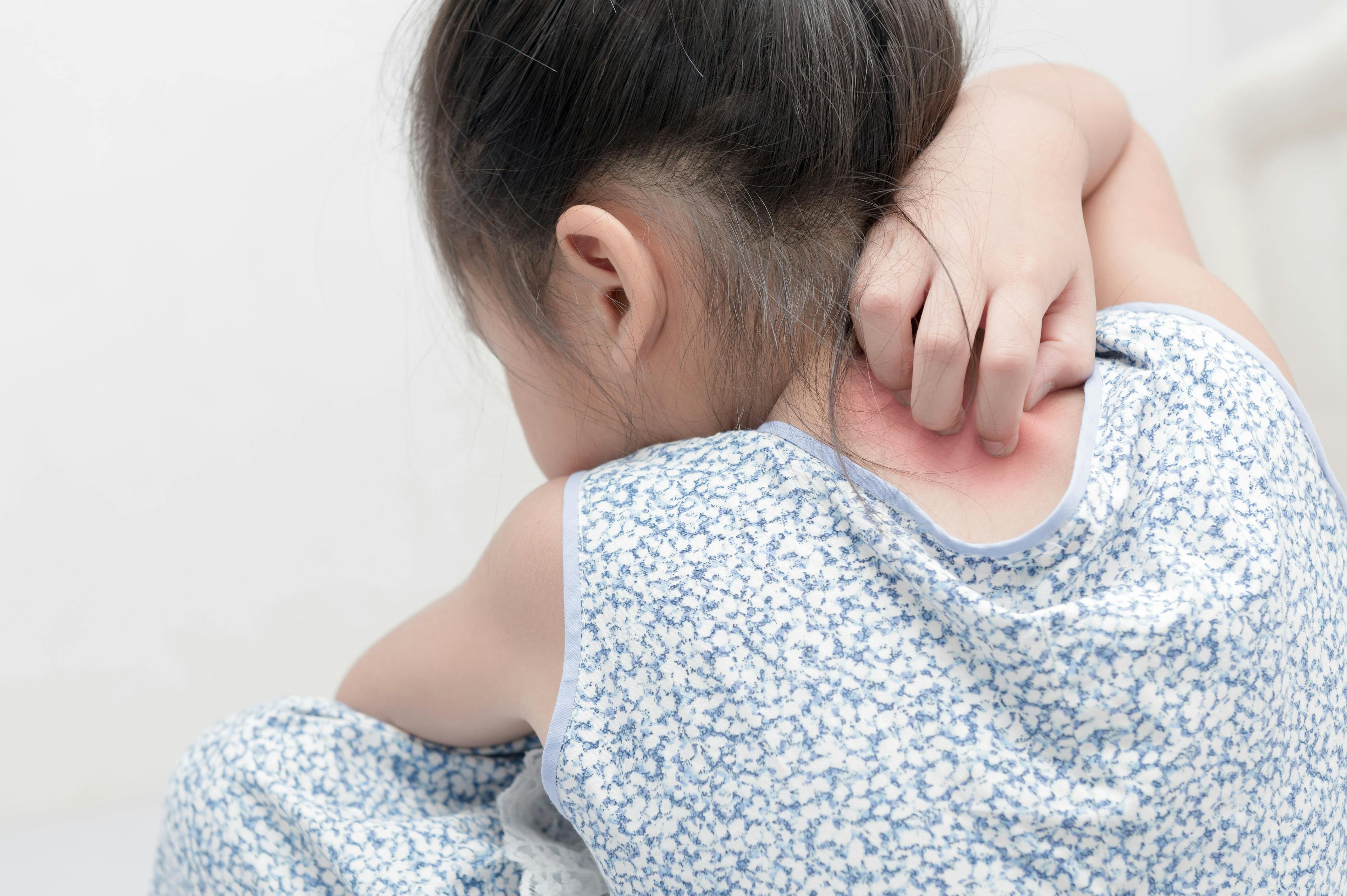
Dupilumab is both efficacious and safe on a long-term basis for pediatric patients with moderate-to-severe atopic dermatitis, according to a poster presented at the Revolutionizing Atopic Dermatitis conference in Washington, DC.1
SkyLine/AdobeStock

Researchers sought to provide efficacy and safety data for dupilumab in pediatric patients in a long-term laboratory setting, citing the frequency of systemic treatments requiring laboratory monitoring, further noting an increased patient treatment burden as a result.
The open-label extension study, LIBERTY AD PED-OLE (NCT02612454), included children ages 6 months to less than 18 years of age with moderate-to-severe atopic dermatitis. In the instance of this study, researchers examined a specific age group of 6 months to 5 years of age.
180 patients were enrolled in the study through completion on a rolling basis. 67.8% of patients completed up to 16 weeks of the study, and 16.7% of patients completed up to 52 weeks.
Patients were treated with dupilumab every 4 weeks, with their assigned dosage being determined by their weight. Patients weighing 5 kg to less than 15 mg were treated with 200 mg of dupilumab, while patients with a baseline weight of 15 kg to less than 30 kg were treated with 300 mg of dupilumab.
For additional comparison of hematologic and chemistry laboratory parameters, researchers also included data from the LIBERTY AD PRESCHOOL part B study. Specifically, they included week 16 data from pediatric patients of the same age range who were treated with dupilumab or a placebo.
Throughout the study’s duration, researchers collected and analyzed data related to patients’ eosinophil and platelet counts, as well as alkaline phosphatase (ALP) and lactate dehydrogenase (LDH) levels. Additionally, they collected further related data points, including counts of hemoglobin, leukocytes, neutrophils, creatine kinase, blood urea nitrogen, and creatine.
Researchers found that overall safety of dupilumab was consistent with previous studies and understandings of the drug’s safety profile. They also noted an average increase in ALP and a decrease in LDH among dupilumab-treated patients that was reflective of a normal range.
Some participants treated with dupilumab experienced adverse events, including mild anemia and thrombocytopenia in the LIBERTY AD PD-OLE patients. In the LIBERTY AD PRESCHOOL patients, some being treated with dupilumab experienced adverse events, including a mild case of neutropenia, 1 moderate case of eosinophilia, and 1 severe case of eosinophilia.
“No clinically meaningful changes in hematologic and chemistry parameters were observed during 52 weeks of dupilumab treatment. While it remained within normal intervals, an increase in mean ALP was observed in dupilumab-treated patients," study authors wrote. “Although eosinophil counts increased slightly from baseline, they decreased below baseline by Week 52. Decreases in mean LDH and platelet counts within the normal range were observed in dupilumab-treated patients, consistent with decreased inflammation. As with adults, adolescents, and older children, routine laboratory monitoring is unnecessary in children aged 6 months to 5 years with moderate-to-severe AD [atopic dermatitis].”
Reference
- Siegfried E, Cork M, Lockshin B, Boguniewicz M, et al. Long-term laboratory safety from open-label extension study of dupilumab in patients aged 6 months to 5 years with moderate-to-severe atopic dermatitis. Poster presented at the 2023 Revolutionizing Atopic Dermatitis Conference; April 29-May 1, 2023; Washington, DC. https://djbpnesxepydt.cloudfront.net/radv/April2023/April-2023-Posters/411_Poster_LT-Dupilumab-Safety_Peds_Siegfried-et-al_1682462290492.pdf

Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.