- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Publication
Article
Dermatology Times
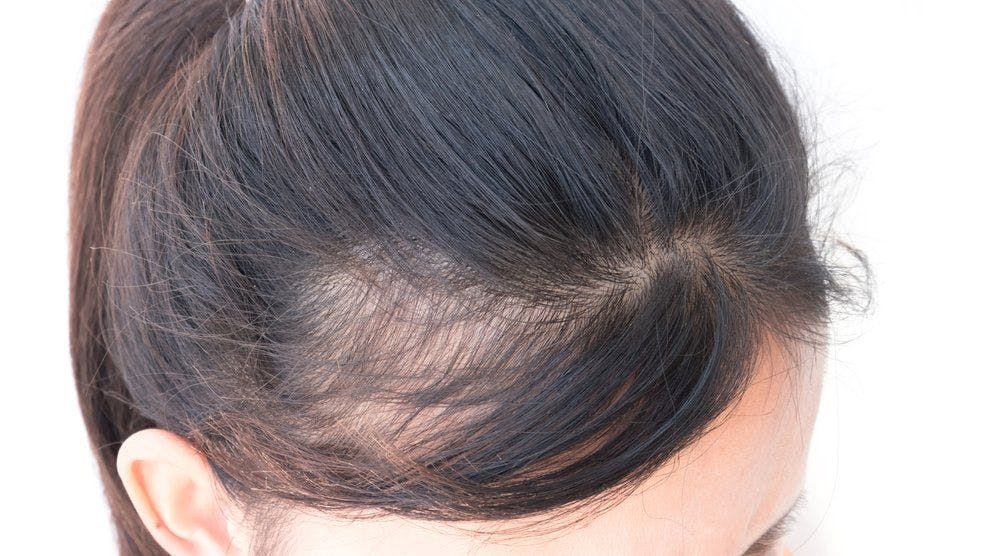
Medical innovation expands hair loss treatment
Author(s):
A variety of topical and injectable therapies are showing promise for addressing the problem of hair loss in both men and women, says Matt Leavitt, D.O.
A variety of topical and injectable therapies are showing promise for addressing the problem of hair loss in both men and women, says Matt Leavitt, D.O.
RELATED: Expert discusses hair loss treatment controversies, concerns
“This is an exciting time in the world of hair restoration. There are many new and emerging products and techniques for the treatment of hair loss,” says Dr. Leavitt, Founder and CEO, Advanced Dermatology and Cosmetic Surgery, Maitland, Fla., who is a hair loss and hair restoration specialist. He is joined at his practice location by Natalie Kash, M.D., who is completing a hair loss and restoration fellowship.
TOPICAL TREATMENTS
Clascoterone (cortexolone 17-alpha proprionate; Breezula, Cassiopea) is an androgen receptor inhibitor that is being developed as a treatment for both male and female androgenetic alopecia (AGA). It is a novel steroid that is rapidly hydrolyzed in plasma to an inactive metabolite. Therefore, it appears to be associated with minimal to no risk of steroid-related systemic side effects.
A phase 2, vehicle-controlled dose-ranging study investigated different concentrations and dosing regimens for clascoterone in males with mild-to-moderate AGA. Its results showed that the treatment had a favorable safety profile and they also provided evidence supporting efficacy. Based on the results, clascoterone 7.5% solution was selected as the best candidate for further study, and a pivotal trial was initiated early in 2020. In addition, a phase 2 study is underway investigating clascoterone in females.
“The results achieved in men in the phase 2 study were pretty dramatic and comparable to some of the best results seen in the past with minoxidil,” Dr. Leavitt says. “More women today are wanting to be treated for hair loss and at an earlier stage, and we often struggle to find safe and effective treatment for women. Oral spironolactone and topical minoxidil have been the primary drugs used for female pattern hair loss, but clascoterone and other topical treatments are showing promise.”
Given the desire for the benefits of finasteride without the potential for systemic side effects, topical use of the anti-androgen has also garnered significant interest. Finasteride blocks the conversion of testosterone to dihydrotestosterone and blocks androgen receptor function. It acts to reduce hair miniaturization and hair inflammation, Dr. Leavitt says.
Findings of a systematic review published in 2018 supported the efficacy of topical finasteride as a treatment for AGA in men and women.1 The review included seven studies, all of which showed that the topical treatment was associated with increased terminal hair counts, decreased rates of hair loss, and positive hair growth assessments.
RELATED: Evaluating hair loss in women
The most common side effects included scalp irritation, erythema and contact dermatitis. There were a number of systemic side effects, but they were rare, and there were no serious side effects.
“The concentrations of finasteride used in these studies varied widely, and there were also variations in the application regimen. In addition, there is evidence that greater efficacy can be achieved by combining topical finasteride with other treatments, including oral finasteride, topical minoxidil and ketoconazole shampoo,” Dr. Leavitt says.“Clearly there is a need for larger studies with longer follow-up to investigate the efficacy and safety of topical finasteride as well as the optimal vehicle, formulation, concentration, and frequency.”
INJECTABLE TREATMENTS
More than 50 studies have reported on injections of platelet rich plasma (PRP) for hair loss. Similar to the situation with topical finasteride, these studies also varied in methodology, including the method for PRP preparation, timing for PRP injection, and outcome measures, Dr. Leavitt says.
“Despite promising results, there are a lot of questions regarding the ideal preparation technique and regimen for using PRP that might provide the best outcomes,” he observes.
There is good biologic rationale for interest in PRP. Because it contains a high concentration of platelets in a small volume of plasma, it represents a rich source of growth factors and cytokines that have been shown to promote vascularization and angiogenesis, trigger and extend anagen, reduce inflammation and oxidative stress, trigger hair stem cell regeneration, activate fibroblasts and stimulate extracellular matrix, Dr. Leavitt says.
Also in the realm of injectable therapy, exosomes are the focus of a lot of attention as an emerging treatment for hair loss. Exosomes are extracellular cell-derived vesicles that transport micro RNA, mRNA, and transcription factors or other proteins from cell to cell, thereby allowing intercellular communication. Their use can promote hair restoration and prevent hair loss via several mechanisms, including by stimulating anti-inflammatory, pro-angiogenic, regenerative and healing pathways.
RELATED: Hair growth treatments
Studies done in animal models showed that exosomes derived from dermal papilla cells promoted anagen, delayed catagen and regulated hair follicle stem cell growth and differentiation. However, there are no basic science data from studies investigating the effects of dermal papilla-derived exosomes and exosomal micro RNA on the human hair follicle and hair follicle stem cells, Dr. Leavitt says.
Currently, several companies are producing exosome products for treating hair loss, although none are FDA-approved.
“There is a need for more clinical data on the long-term efficacy and safety of this approach,” he says.
Disclosures:
Dr. Leavitt is Executive Medical Advisor for Bosley. He has received no financial support, grants and/or research support regarding clascoterone, topical finasteride, minoxidil, spironolactone, ketoconazole, exosomes or platelet rich plasma (PRP) or any companies associated with these medications/therapies.
References:
1. Lee SW, Juhasz M, Mobasher P, Ekelem C, Mesinkovska NA. A Systematic Review of Topical Finasteride in the Treatment of Androgenetic Alopecia in Men and Women. J Drugs Dermatol. 2018;17(4):457-463.

Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.