- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Publication
Article
Dermatology Times
April Armstrong, MD, MPH, Reviews New Treatment Options for Patients With Psoriasis
Author(s):
Topicals, orals, biologics—Armstrong’s Winter Clinical Miami presentation focused on new treatment options for patients with psoriasis with an emphasis on real-world observations.
(Adobe Stock/Milan Lipowski)
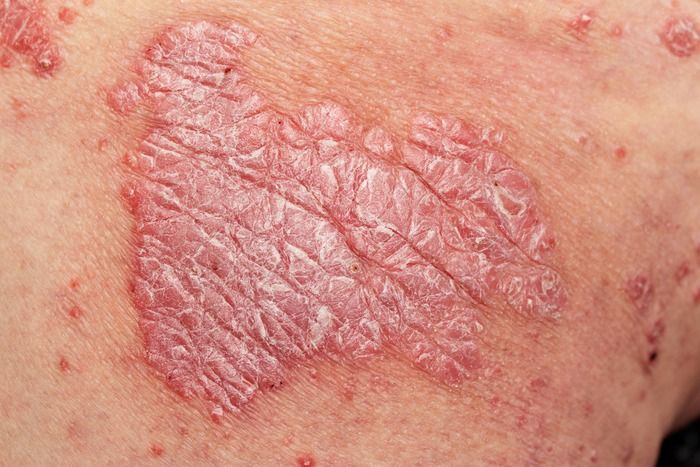
Clinicians already have a number of options at their disposal when considering treatment for a patient with psoriasis, but how do they decide which to use when, and how do newer drugs stack up against the tried-and-true therapies?
April Armstrong, MD, MPH, professor of dermatology and associate dean of clinical research at University of Southern California, sought to answer this question at Winter Clinical Miami, held February 17-20, 2023, in Miami, Florida, with her presentation, “What Is New That We Can Use for Our Psoriasis Patients?”1
Armstrong first summarized the evolution of topical therapies for psoriasis, starting with arsenic and coal tar through to steroids and topical vitamin D. Two non-steroidal agents that recently gained FDA approval to treat patients with plaque psoriasis are tapinarof, an aryl hydrocarbon receptor agonist, and roflumilast, a phosphodiesterase 4 (PDE4) inhibitor.
Tapinarof, Armstrong explained, is a small molecule that travels inside the cell and binds to the aryl hydrocarbon receptor. Effectively, it decreases Th17 cytokines; boosts antioxidant activity (a feature that is relatively novel in topical therapies); increases filaggrin, loricrin, and involucrin proteins; and decreases Th2 cytokines. This results in less inflammation, less oxidative stress, increased skin barrier normalization, and a decrease in inflammation associated with atopic dermatitis. That is why, Armstrong said, tapinarof will likely soon gain an additional indication for atopic dermatitis.
In the 2 pivotal phase 3 studies that led to its approval, PSOARING 1 and PSOARING 2 (NCT03956355 and NCT03983980), between 35% and 40% of tapinarof-treated patients achieved clear or almost clear skin by month 3. One interesting observation about the topical that was evident in the clinical trials, Armstrong noted, is that patients who were treated to clearance, who then stopped treatment, maintained their skin clearance.
“They actually hold their response for a period of time…they hold it for about 4 months, so they can actually not apply anything and still maintain clear or almost clear,” she said during her lecture. “I thought that was probably a little bit more ‘real-world’…Our patients, typically with topical therapies, they'll apply it when they see something [and then] they stop when they don't, so it's good to see that the non-steroidals can potentially hold that beneficial effect for a while.”
The second non-steroidal topical for plaque psoriasis that Armstrong mentioned was roflumilast, which has shown to be more potent than apremilast, although the 2 have a similar mechanism of action. Like tapinarof, it is also applied as a once-daily application that makes it convenient for patients.
In terms of efficacy, at 8 weeks roflumilast resulted in clear or almost clear with at least a 2-grade improvement from baseline for 42.4% and 37.5% of patients in the phase 3 studies supporting its approval, DERMIS-1 (NCT04211363) and DERMIS-2 (NCT04211389), respectively, compared with vehicle treatment. Notably, Armstrong said, it can be used in the intertriginous areas with decent efficacy.
Moving over to oral medications, the new kid on the block is deucravacitinib, a tyrosine kinase 2 (TYK2) inhibitor with a novel mechanism of action.
“We know that IL-23 is very important for psoriasis, but how does IL-23 transmit its signal? It transmits its signal by binding to the receptor. But in order for that signal to be transmitted in the cell, it actually needs a TYK2 binding to JAK-2 with the receptor,” Armstrong explained. “With this binding, that's how you actually attract intracellular STAT molecules and they go to the nucleus, and they do their thing. TYK2 is very important in this transmission of signaling of IL-23.”
Deucravacitinib has a very well-tolerated safety profile and demonstrated superiority compared with apremilast in 2 phase 3 head-to-head studies, POETYK PSO-1 (NCT03624127) and POETYK PSO-2 (NCT03611751). Additionally, long-term maintenance data demonstrate efficacy with upwards of 80% of patients achieving Psoriasis Area and Severity Index (PASI) 75 response at 2 years. The drug is currently in phase 3 clinical trials for psoriatic arthritis, as well, among other indications.
Armstrong closed with a brief discussion on generalized pustular psoriasis (GPP), which she described as a “very emergent” condition that is more prevalent in the Asian and female populations.
“Treating GPP has been almost impossible in the past, until now…hope is here,” she told the audience. “Essentially, we have learned that in GPP, it's the dysregulation in the IL-36 pathway. You have too much IL-36 agonist because the patient is lacking an intrinsic IL-36 antagonist. What happens is that when you have this elevated level of IL-36 agonist, you get tremendous increase in the cytokines.”
Newly approved spesolimab blocks IL-36 receptor and results in significant improvement with just one intravenous dose, Armstrong said.
Reference:
Armstrong A. What is new that we can use for our psoriasis patients? Presented at Winter Clinical Miami 2023; February 17-20, 2023; Miami, FL.

Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.