- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Publication
Article
Dermatology Times
Phase 3 results show apremilast safe, effective for plaque psoriasis
Author(s):
Amgen releases positive phase 3 results evaluating U.S. FDA-approved apremilast (Otezla) for the treatment of mild-to-moderate plaque psoriasis in adults.
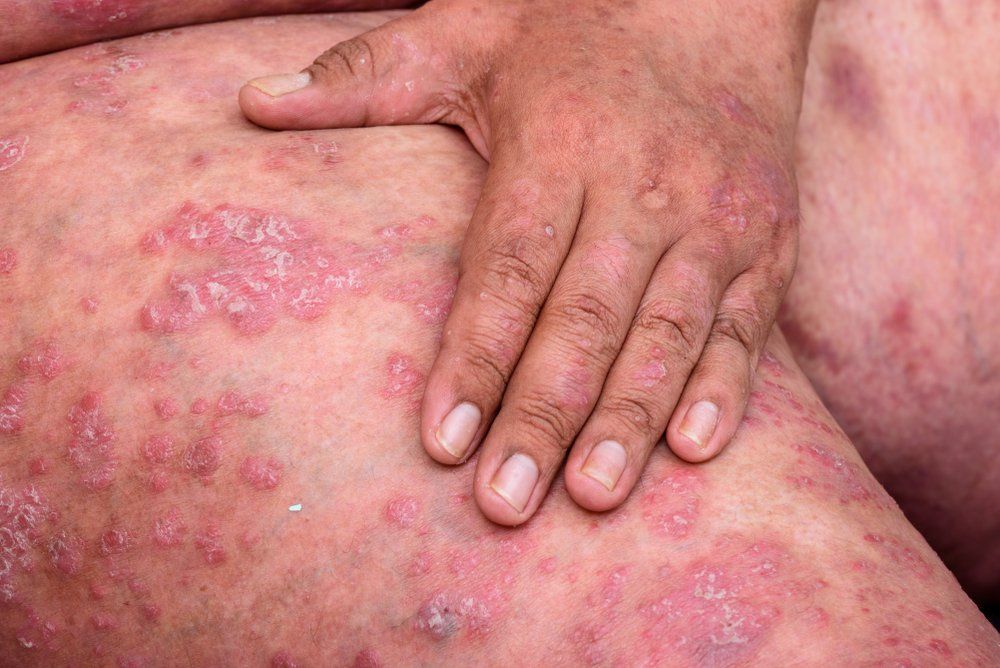
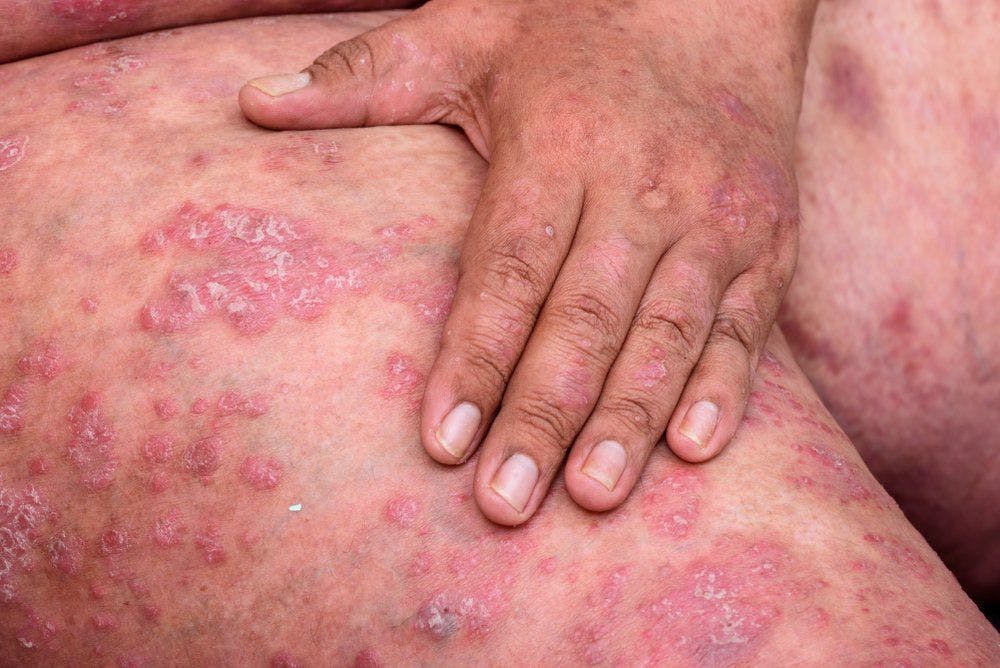
Apremilast (Otezla, Amgen), a U.S. Food and Drug Administration (FDA)-approved phosphodiesterase 4 (PDE4) inhibitor for treatment of mild-to-moderate plaque psoriasis in adults, was recently subject of a phase 3 clinical trial investigating its safety and efficacy.
The ADVANCE study is a double-blind, multicenter, placebo-controlled trial that examined apremilast in 595 adult subjects with mild-to-moderate plaque psoriasis who were given 30 mg of Apremilast or placebo twice daily for 16 weeks then followed by an open-label extension study that extended to week 32.
MORE: Meta-analysis points to most effective biologics for psoriasis
Results of the study demonstrated a positive outcome with apremilast achieving its primary end point of a statically significant improvement in Static Physicians Global Assessment (sPGA) response at week 16 versus placebo. Secondary end points in the study were also met with a minimum of 75% improvement from baseline in percent of affected body surface area (BSA), an alteration in Psoriasis Area and Severity Index (PASI) score from baseline and a change in BSA score from baseline.1
Safety was also evaluated in the study with the safety profile mimicking those in previous studies. The most common treatment-related adverse events (≥5%) include nausea, headache, nasopharyngitis, upper respiratory tract infection and diarrhea.
RELATED: Psoriasis guidelines emphasize patient education, comorbidities
"Many patients with mild-to-moderate plaque psoriasis who use topical therapies still have challenges managing their psoriasis," says David M. Reese, M.D., executive vice president of research and development at Amgen. "We look forward to discussions with the FDA about the potential to bring Otezla, which has already been prescribed to hundreds of thousands of patients with moderate-to-severe psoriasis, to more patients who may need additional therapeutic options."
More details on the study can be found at drugtopics.com.
References:
Amgen Announces Positive TopLine Results From Otezla apremilast Phase 3 ADVANCE Study In MildToModerate Plaque Psoriasis. Amgen, Inc. https://www.amgen.com/media/news-releases/2020/05/amgen-announces-positive-topline-results-from-otezla-apremilast-phase-3-advance-study-in-mildtomoderate-plaque-psoriasis/. Published May 6, 2020. Accessed May 11, 2020.

Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.