- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Publication
Article
Dermatology Times
Continuing Medical Education: 3 Things You Should Know About Managing Alopecia Areata
Author(s):
Alopecia areata can have devastating effects on patients, but a greater understanding of its pathophysiology has led to the development of new treatment options.
Alopecia areata (AA) is a chronic autoimmune condition that damages the hair follicles. It typically presents with sharply defined round patches of nonscarring hair loss on the scalp.1 However, it can also affect the eyebrows, lashes, facial hair, and nails, and it can progress to alopecia totalis (total scalp hair loss) or alopecia universalis (total body hair loss). AA can have devastating effects on patients, but a greater understanding of its pathophysiology has led to the development of new treatment options. Here are 3 things you should know about managing alopecia areata.
1. AA can have a profound psychosocial impact.
With a lifetime incidence risk of approximately 2%, AA is the second most common cause of hair loss, behind androgenetic alopecia, and it affects men and women comparably.2-4 Up to 88% of patients experience their initial episode of AA prior to age 40 years, with spontaneous remission rates between 30% and 50% within the first 6 to 12 months of onset.2,5 Overall, the incidence of relapse is 85% and approaches 100% over 20 years.
Patients with AA have a higher prevalence of depression, anxiety, and sleep problems than the general population.6,7 In a cross-sectional online survey study, 62% of patients said AA caused them to make different major life decisions with respect to relationships, education, or career.8 Additionally, 85% said that it is a daily challenge to cope with AA, and 47% reported anxiety and/or depression.8 Results of a systematic review showed that patients with AA consistently exhibit poor health-related quality of life, similar to patients with other chronic dermatologic diseases, including atopic dermatitis and psoriasis.9
The concerns of stigma in patients with AA may not be unfounded. A survey of 2015 laypersons who were shown 3 images of the same person with varying amounts of hair found that 30% of respondents thought of those with severe hair loss as sick, and 27% viewed them as unattractive. Stigma increased as degree of hair loss increased.10
2. The pathogenesis of AA is gaining new insights.
The normal human hair growth cycle consists of 3 phases: anagen (active hair/follicular growth), catagen (regression/follicular involution), and telogen (follicular rest and shedding).11 In AA, autoimmune-mediated damage to the hair follicle in the anagen phase leads to premature transition to the catagen and telogen phases and subsequently to loss of hair.
The anagen hair follicle normally has immune privilege due to production of local immunosuppressants and downregulation of major histocompatibility complex class I proteins, among other factors.12 Breakdown of immune privilege of the hair follicle (resulting from genetic, environmental, and immunologic factors) is thought to be a key element in the pathophysiology of AA.13 With the collapse of immune privilege, autoreactive CD8-positive and natural killer (NK) group 2D–positive (NKG2D+) T cells can attack follicular epithelial cells.14
When these T cells bind to autoantigens at the follicle, they release the cytokine interferon-γ (IFN-γ), which is a key driver of AA pathogenesis.15 In response to IFN-γ, follicular epithelial cells then signal via the Janus kinase (JAK)/signal transducer and activator of transcription (JAK-STAT) pathway to upregulate γ-chain cytokines, including interleukin (IL)-2 and IL-15.16 IL-2 and IL-15 are known drivers of cytotoxic T-cell activity.17 JAK-mediated production of IL-15 promotes further inflammation and T-cell activation, causing additional release of IFN-γ via JAK signaling in a positive feedback loop. These cytokines and chemokines further recruit CD4-positive T cells and natural killer cells to the hair follicle, leading to dystrophy.18
3. The management of AA is individualized.
AA has historically been treated with intralesional or topical corticosteroids.11 However, the efficacy of these treatments is limited, and few randomized controlled trials have been performed. Additionally, safety concerns of local corticosteroids include telangiectasias and skin atrophy. In a cross-sectional online survey study, patient-cited reasons for discontinuing AA treatment included adverse effects and lack of efficacy.8
Newer agents in development target the cytokines involved in AA pathogenesis. JAK inhibitors are small-molecule drugs that block JAK enzymatic activity, thereby blocking cytokine signaling and STAT activation.19 This is thought to prevent the upregulation of IFN-γ. Tofacitinib, a first-generation JAK inhibitor, has been reported to improve hair growth in patients with AA in many small case-report and case-series studies, but a large randomized controlled trial is lacking.11 The Table20-24 provides a summary of other JAK inhibitors studied for use in AA.
Table: Select JAK Inhibitors Studied for Use in Alopecia Areata20-24
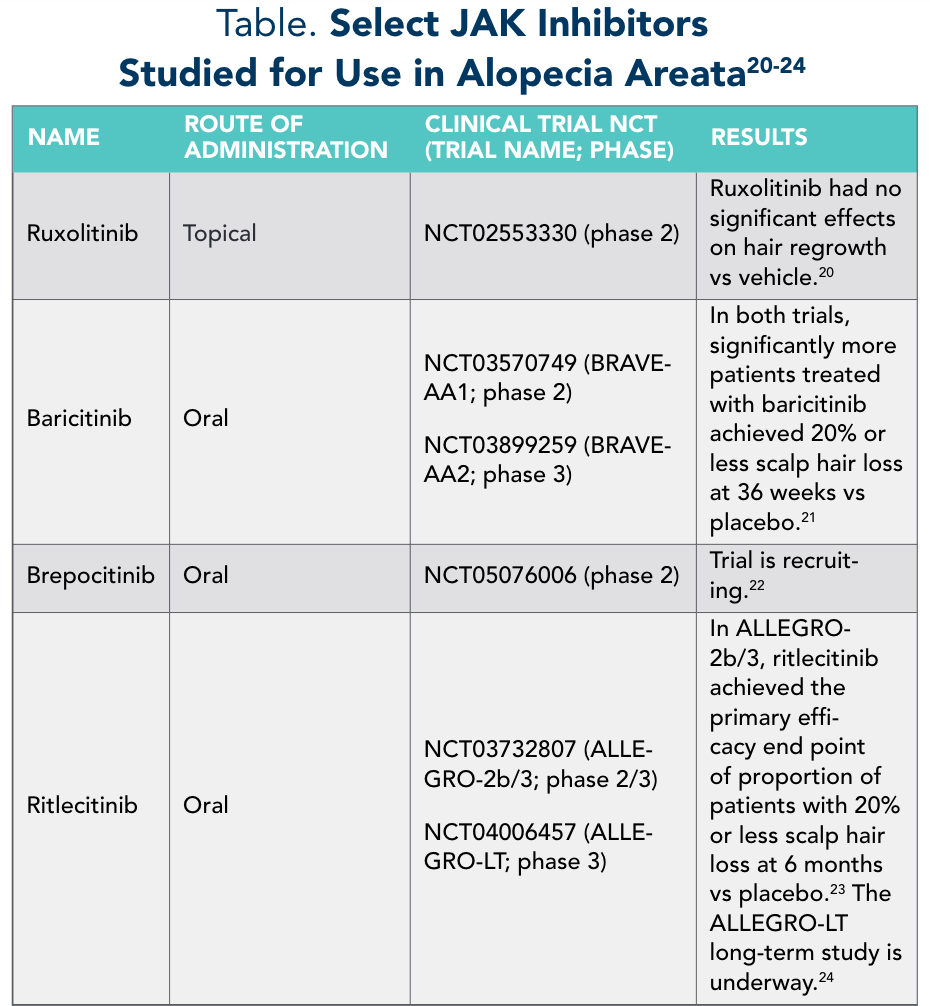
Baricitinib is currently the only FDA-approved JAK inhibitor for AA.25 A meta-analysis of the use of tofacitinib, ruxolitinib, and baricitinib in patients with AA found that 72% of 289 cases responded to therapy, with a mean time to hair growth of 2 months.26
Treatment algorithms for AA are based on patient age, disease activity and severity, and treatment response.27 When managing patients with AA, it is important to consider a shared decision-making approach—taking into account disease severity, quality of life, comorbidities, and long-term efficacy and safety—to provide the best possible treatment and improve patient outcomes.
Key References:
8. Mesinkovska N, King B, Mirmirani P, Ko J, Cassella J. Burden of illness in alopecia areata: a cross-sectional online survey study. J Investig Dermatol Symp Proc. 2020;20(1):S62-S68. doi:10.1016/j.jisp.2020.05.007
11. Dillon KL. A comprehensive literature review of JAK inhibitors in treatment of alopecia areata.Clin Cosmet Investig Dermatol. 2021;14:691-714. doi:10.2147/ccid.S309215
26. Rattananukrom T, Suchonwanit P. Are drug treatment strategies really effective against alopecia areata? Expert Opin Pharmacother. 2021;22(3):257-260. doi:10.1080/14656566.2020.1854728
For a full list of references go to www.gotoper.com/aad22iss-art
Disclosures:
This activity was written by Physicians’ Education Re-source®, LLC (PER®), editorial staff under faculty guidance and review.
Faculty, Staff, and Planners’ Disclosures
In accordance with ACCME Guidelines, PER® has identified and resolved all COI for faculty, staff, and planners prior to the start of this activity by using a multistep process.
Disclosures (Dr King):
Consultant: AbbVie, AltruBio, Almirall, AnaptysBio, Arena Pharmaceuticals, Bioniz Thera-peutics, Bristol Myers Squibb, Concert Pharmaceuticals, Equillium, Horizon Therapeutics, Incyte, Janssen Pharmaceuticals, LEO Pharma, Eli Lilly and Company, Otsuka/Visterra, Pfizer.
The staff of Physicians’ Education Resource®, LLC, have no relevant financial relationships with ineligible companies.
To learn more about this topic, including information on treating patients with alopecia areata, go to www.gotoper.com/aad22iss-act
Release Date: October 1, 2022
Expiration Date: October 1, 2023
Learning Objectives Upon successful completion of this activity, you should be better prepared to:
- Identify potential therapeutic targets for alopecia areata
- Outline management plans to optimize outcomes and quality of life for patients with alopecia areata.
Accreditation/Credit Designation
Physicians’ Education Resource®, LLC, is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians.
Physicians’ Education Resource®, LLC, designates this enduring material for a maximum of 0.25 AMA PRA Category 1 Credits™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Physicians’ Education Resource®, LLC, is approved by the California Board of Registered Nursing, Provider #16669, for 0.25 Contact Hours.
Acknowledgment of Commercial Support
This activity is supported by an educational grant from Pfizer Inc.
Off-Label Disclosure/Disclaimer
This activity may or may not discuss investigational, unapproved, or off-label use of drugs. Learners are advised to consult prescribing information for any products discussed. The information provided in this activity is for accredited continuing education purposes only and is not meant to substitute for the independent clinical judgment of a health care professional relative to diagnostic, treatment, or management options for a specific patient’s medical condition. The opinions expressed in the content are solely those of the individual faculty members and do not reflect those of PER® or any company that provided commercial support for this activity.
Instructions for Participation/How to Receive Credit
1. Read this activity in its entirety.
2. Go to www.gotoper.com/aad22iss-art to access and complete the posttest.
3. Answer the evaluation questions.
4. Request credit using the drop-down menu. You may immediately download your certificate.
CME POST TEST QUESTIONS


Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.