- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Publication
Article
Dermatology Times
Overview of Prurigo Nodularis for Practicing Dermatology Clinicians
Author(s):
It is crucial to better understand, diagnose, and manage this skin condition associated with significant quality-of-life impairment.
Prurigo nodularis (PN) is a chronic inflammatory skin condition characterized by intense pruritis, taking the form of firm, hyperplastic, intensely itchy nodules often found on extensor surfaces of the limbs and trunk. Although exact pathogenesis is still unclear, PN has been shown to be associated with neural and immune dysregulation mediated by inflammatory cytokines and neuro-peptides, leading to the condition’s persistent, severe itch. In part due to lack of understanding of pathophysiology, there are no FDA-approved treatments. Given the condition’s detrimental impact on quality of life, it is critical to better diagnose, manage, and understand PN, as well as the itch and pain associated with significant morbidity.
Variation in Presentation
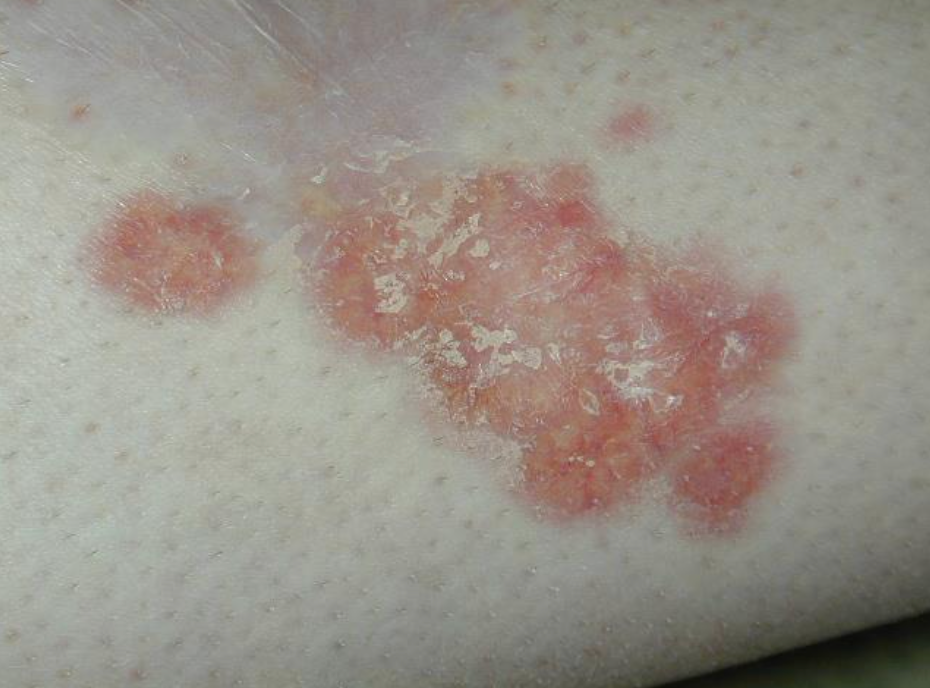
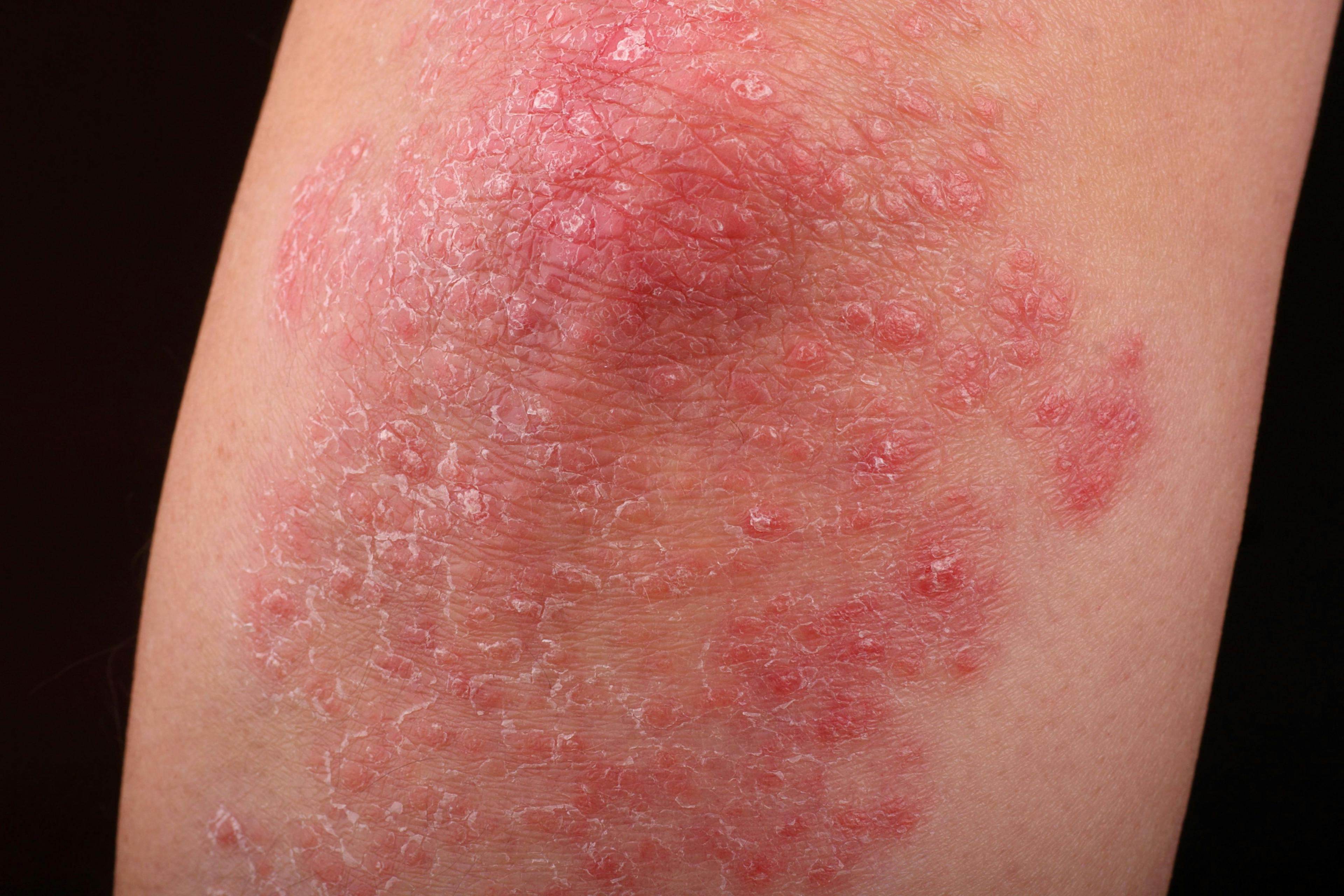
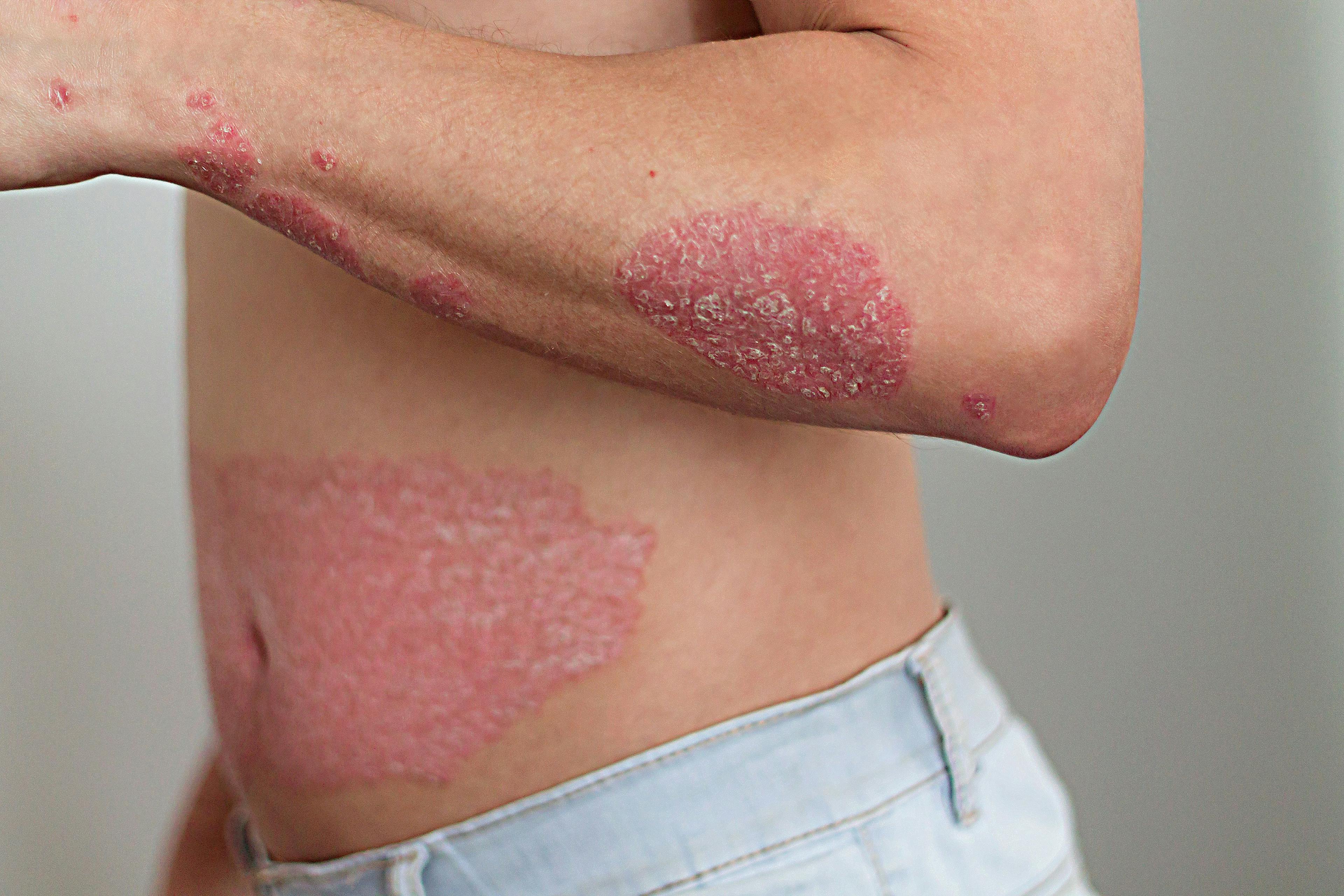
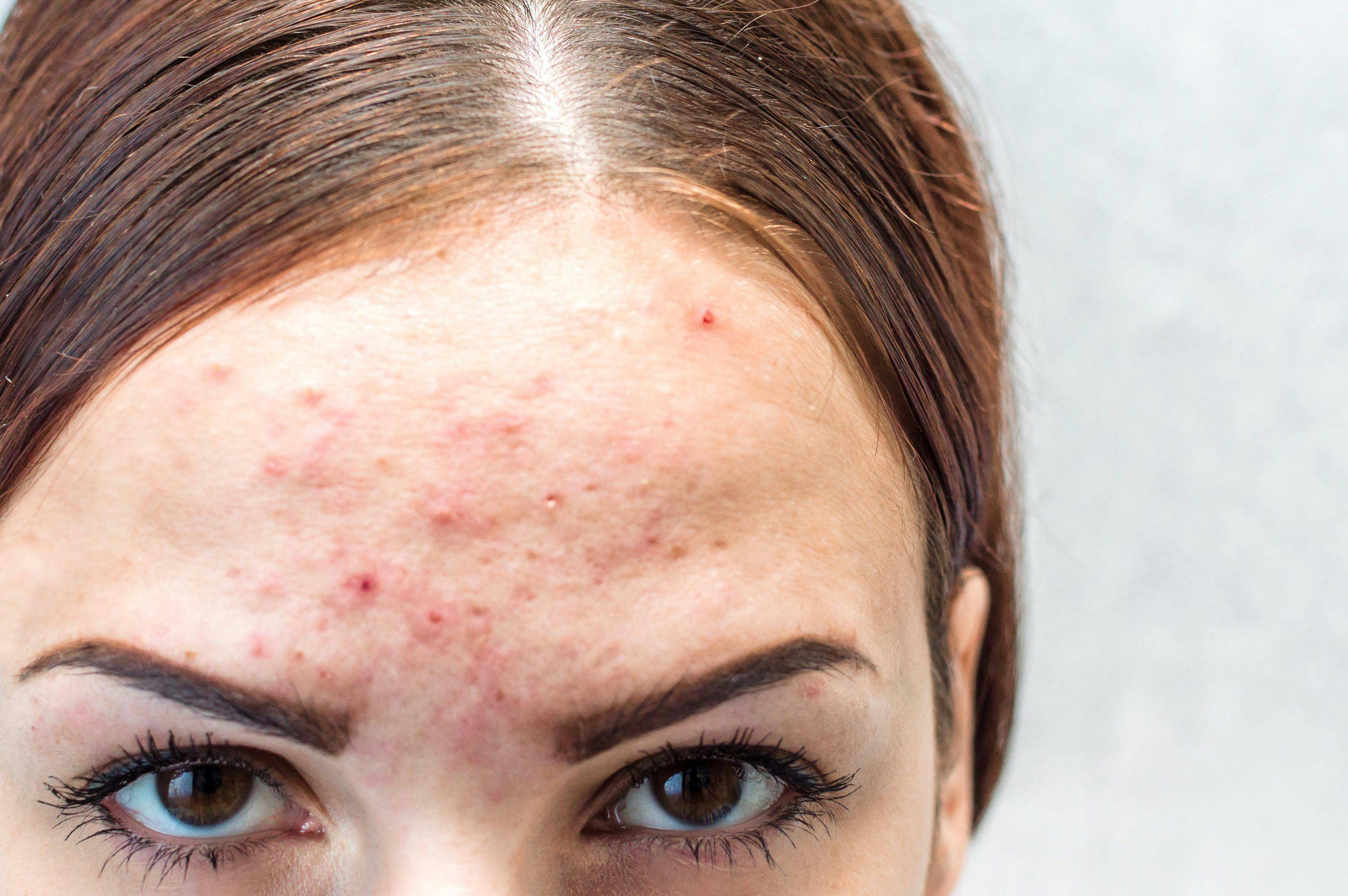
A patient with PN will typically present with firm nodules symmetrically distributed in groups on extensor surfaces of the arms, legs, and trunk. Notably, lesions typically spare the middle upper back in what is termed the “butterfly” sign. Nodule appearance can vary—typically measuring a few millimeters to 2 cm, ranging from flesh-colored to pink to brown to black, and numbering a few to hundreds.1,2 Skin color may also affect presentation; for Black patients, for example, PN nodules may be firmer, larger, and darker.
Repeated itching and mechanical scratching frequently leads to thickening, crusting, and excoriation of the nodules over time, which can lead to scarring as nodules heal.3 Most patients report severe itching accompanied by sensations of pain, stinging, and burning in both nodular and internodular skin.2 The itch, pain, and stress can significantly impact quality of life and health care utilization. Aggarwal et al found that patients with PN had mean scores of 16.4, 11.6 and 16.8 on the Dermatology Life Quality Index, Pittsburgh Sleep Quality Index, and 5-Dimensions Itch scale, respectively, as well as a high medical-appointment bur-den, with 36.3% of 231 surveyed patients reporting 10 or more doctor visits in the past year.2
Epidemiology
PN is relatively rare, with an estimated prevalence of 22 to 72 per 100,000 adults.4,5 These estimates, however, may be conservative due to inconsistency in PN coding. Additionally, more work needs done to understand prevalence in the pediatric population.4 In the adult population, PN commonly affects middle-aged patients in the fifth and sixth decades of life, with an epidemiologic study of 7095 adult patients suggesting that the diagnosis is more common in women than men (53.1% vs. 46.9 %).6
Patients with skin of color are also at increased risk, as shown by both single-institution and national database research. Compared with White patients, African American patients are roughly 3.4 times more likely to have PN.7 There is also increased burden for inpatient stays for African American, Asian, and Hispanic patients with PN.8 Factors such as inadequately treated atopic dermatitis and barriers in health care access may increase the risk of concomitant PN in patients of color.
Studies comparing PN with other inflammatory skin conditions have also shown a higher burden of systemic comorbidities with PN. Given the complex immune dysregulation associated with PN, it may come as little surprise that PN is associated with other dermatologic diseases, most commonly atopic dermatitis as well as psoriasis, neurotic excoriations, keratoacanthomas, and bullous pemphigoid. In addition to dermatologic conditions, PN is also linked to multiorgan systemic diseases spanning infection, metabolic dysfunction, and malignancy. Notably, patients with PN have 2.68 higher odds of HIV infection and 2 to 5 times increased odds of non-Hodgkin lymphoma when compared with controls.4
Other common copresenting conditions include celiac disease, thyroid disease, diabetes, multiple myeloma, primary cutaneous lymphoma, and end-stage renal disease. Patients with PN also have increased rates of mood and anxiety disorders vs age- and sex-matched controls and other patients with atopic dermatitis and psoriasis.6 There are also increased odds of self-harm, eating disorders, schizophrenia, and other mental health conditions.6,9
Although more research is needed to clarify causal relationship between PN and copresenting conditions, the diverse range of comorbidities may promote the itch-scratch cycle and PN worsening.
Diagnosis
PN is a primarily clinical diagnosis based on history and physical examination. Key findings include the presence of symmetrical, firm, nodular lesions on the extensor limbs and trunk of the body that spare the middle upper back (butterfly sign), pruritus lasting longer than 6 weeks, and history of repeated scratching of lesions. Other conditions that may present similarly and trigger PN include insect bites, scabies surrepticius (mistaken for other dermatologic diagnoses), lupus erythematosus, multiple keratoacanthomas, atopic dermatitis, and psoriasis vulgaris. Although manifestations in adults are better understood, PN in adolescents and children may present similarly but be more commonly associated with atopic dermatitis in otherwise healthy individuals.10 Differential diagnoses that present less commonly include pemphigoid nodularis, actinic prurigo, epidermolysis bullosa, hypertrophic lichen planus, and neurotic excoriations.10 Skin biopsy of lesional sites may be used to help distinguish PN from these conditions. Characteristic histologic features include orthohyperkeratosis, parakeratosis, hypergranulosis, and acanthosis in the epidermis. The dermis is fibrotic with inflammatory infiltrate and vertical streaking of collagen bundles in the papillary dermis.
Because PN is often associated with systemic etiologies, including HIV, hepatitis C, liver disease, renal failure, and thyroid disease, laboratory work-up should be completed for all patients with PN. A thorough laboratory evaluation may be comprised of a complete blood count with differential; complete metabolic panel; hemoglobin A1C; HIV antibody test; hepatitis serologies; and liver, kidney, and thyroid function tests.1
Treatment
Treatment of PN can be challenging because the off-label therapies used have variable efficacy in reducing pruritus. Therapies target the immunologic and neural components of PN, primarily aiming to reduce inflammation and neural dysregulation. Topical treatments and phototherapy are commonly used, but often systemic treatments are required to achieve control of the condition.3
Topical treatments include corticosteroids, calcineurin inhibitors, calcipotriol, anesthetics, and capsaicin. Topical corticosteroids to reduce inflammation, often betamethasone valerate 0.1%, may be used with or without occlusion to improve absorption.11 Intralesional injections of corticosteroids, often triamcinolone acetonide, are also used and may be required to penetrate thicker lesions. Efficacy may be further increased by use of adjunctive cryotherapy.12 Calcineurin inhibitors, such as pimecrolimus 1% and tacrolimus 0.1%, have similar efficacy to topical corticosteroids and may be used long term without the adverse effect profile of corticosteroids.13 Calcipotriol ointment, a synthetic derivative of vitamin D, has also shown similar efficacy to betamethasone valerate in a randomized controlled trial.12 To address the neural component of PN, topical anesthetics or topical capsaicin may be used, although these treatments have limited evidence supporting their efficacy.11
Phototherapy is frequently used in conjunction with topical treatments because it has proved to be effective in many studies and has minimal adverse effects. Both psoralen plus UV-A (PUVA) and narrowband UV-B are used with similar success, although a trial of 22 patients with PN showed that using both PUVA and UV-B excimer light in combination reduces the number of PUVA sessions required to achieve complete or almost complete remission of PN.13
Systemic treatments are often required, as many patients with PN are refractory to topical treatments and phototherapy. Immunomodulators used for PN include oral cyclosporine and methotrexate, although they must be used carefully due to their adverse effect profiles and patients should be monitored throughout use.13 Neuromodulatory treatments include gabapentinoids, antidepressants, opioid receptor agonists/antagonists, neurokinin-1 receptor (NK1R) antagonists, and thalidomide. Gabapentinoids, such as gabapentin and prega-balin, target calcium signaling in itch. However, providers should be mindful of the risk of sedation in patients with higher doses of gabapentinoids and may need to slowly titrate treatment upward. Antidepressants, including paroxetine, duloxetine, amitriptyline, fluvoxamine, and mirtazapine, also have reduced itch in small studies of patients with PN.11 Because dysregulation in activation of opioid receptors has been implicated in itch, the μ-opioid receptor antagonists naltrexone and naloxone have been effective in case series in reducing itch and improving lesions.12 Oral nalbuphine ER (Haduvio), a mixed κ-opioid receptor agonist/μ-opioid receptor antagonist also shows promise in phase 2b/3 clinical trials.14 Aprepitant and serlopitant are NK1R antagonists that target substance P, a neuropeptide shown to be elevated in PN lesions. They have previously shown success in small studies but failed to show efficacy vs placebos in larger randomized controlled trials. Finally, thalidomide may be used for patients refractory to treatment but should be prescribed carefully due to its terato-genic and neurotoxic effects.11
Several novel therapies are in development for treatment of PN. Dupilumab, a biologic that targets the IL-4 receptor, is already approved by the FDA for atopic dermatitis and elicited clinically significant reduction in itch in 60% of patients with PN vs 18% of patients with PN treated with placebo.15 Nemolizumab, another biologic, targets the IL-31 receptor and also elicited significant reduction in itch in 56% of patients with PN vs 21% of patients with PN treated with placebo.16 Vixarelimab, which targets oncostatin M receptor β, and barzolvolimab, which targets the mast cell KIT receptor, are also promising biologics in phase 2 and phase 1 clinical trials for PN, respectively. Finally, abrocitinib is a JAK1 inhibitor that targets several inflammatory pathways and is in phase 2 clinical trials.17
Many of these systemic therapies are also in development for other primary dermatologic disorders such as atopic dermatitis and psoriasis, which may create opportunities for treatment synergy given the high degree of PN comorbidity. With further research, these treatment options hold great promise for providing more reliably effective treatments for patients with PN.
References
1. Kwon CD, Khanna R, Williams K A, Kwatra MM, Kwatra SG. Diagnostic workup and evaluation of patients with prurigo nodularis. Medicines (Basel). 2019;6(4):97. doi:10.3390/medicines6040097
2. Aggarwal P, Choi J, Sutaria N, et al. Clinical characteristics and disease burden in prurigo nodularis. Clin Exp Dermatol. 2021;46(7):1277-1284. doi:10.1111/ced.14722
3. McColl M, Boozalis E, Aguh C, Eseonu AC, Okoye GA, Kwatra SG. Pruritus in Black skin: unique molecular characteristics and clinical features. J Natl Med Assoc. 2021;113(1):30-38. doi:10.1016/j.jnma.2020.07.002
4. Huang AH, Williams K A, Kwatra SG. Prurigo nodularis: epidemiology and clinical features. J Am Acad Dermatol. 2020;83(6):1559-1565. doi:10.1016/j.jaad.2020.04.183
5. Ständer S, Augustin M, Berger T, et al. Prevalence of prurigo nodularis in the United States of America: a retrospective database analysis. JAAD Int. 2021;2:28-30. doi:10.1016/j.jdin.2020.10.009
6. Huang AH, Canner JK, Khanna R, Kang S, Kwatra SG. Real-world prevalence of prurigo nodularis and burden of associated diseases. J Invest Dermatol. 2020;140(2):480-483.e4. doi:10.1016/j.jid.2019.07.697
7. Boozalis E, Tang O, Patel S, et al. Ethnic differences and comorbidities of 909 prurigo nodularis patients. J Am Acad Dermatol. 2018;79(4):714-719.e3. doi:10.1016/j.jaad.2018.04.047
8. Whang K A, Kang S, Kwatra SG. Inpatient burden of prurigo nodularis in the United States. Medicines (Basel). 2019;6(3):88. doi:10.3390/medicines6030088
9. Han J, Palomino A, Estupinan B, Wozniak A, Swan J. Psychiatric comorbidity in prurigo nodularis and the impact of socioeconomic status. J Clin Aesthetic Dermatol. 2022;15(6):53-58.
10. Murakami Y, Sugiyama A, Ideguchi H, et al. Three cases of atopic dermatitis children with intractable prurigo nodularis. Arerugi. 2020;69(3):213-217. doi:10.15036/arerugi.69.213
11. Williams K A, Huang AH, Belzberg M, Kwatra SG. Prurigo nodularis: pathogenesis and management. J Am Acad Dermatol. 2020;83(6):1567-1575. doi:10.1016/j.jaad.2020.04.182
12. Kowalski EH, Kneiber D, Valdebran M, Patel U, Amber KT. Treatment-resistant prurigo nodularis: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:163-172. doi:10.2147/CCID.S188070
13. Elmariah S, Kim B, Berger T, et al. Practical approaches for diagnosis and management of prurigo nodularis: United States expert panel consensus. J Am Acad Dermatol. 2021;84(3):747-760. doi:10.1016/j.jaad.2020.07.025
14 . Trevi Therapeutics reports positive results from the ph2b/3 PRISM trial of Haduvio in the treatment of prurigo nodularis. News release. Trevi Therapeutics Inc. June 29, 2022. Accessed September 1, 2022. https://w w w.prnewswire.com/news-releases/trevi-therapeutics-reports-positive-results-from-the-ph2b3-prism-trial-of-haduvio-in-the-treatment-of-prurigo-nodularis-301577632.html
15. Second positive phase 3 Dupixent (dupilumab) trial confirms significant improvements for patients with prurigo nodularis. News release. Sanofi. Januar y 19, 2022. Accessed September 1, 2022. https://www.sanofi.com/en/media-room/press-releases/2022/2022-01-19-06-00-00-2368986
16. Galderma announces positive data from phase III trial, demonstrating efficacy and safet y of nemolizumab in patients with prurigo nodularis. News release. Galderma. June 22, 2022. Accessed September 1, 2022. https://w w w.galderma.com/news/galderma-announces-positive-data-phase-iii-trial-demonstra-ting-efficacy-and-safet y-nemolizumab
17. Labib A, Ju T, Vander Does A, Yosipovitch G. Immunotargets and therapy for prurigo nodularis. Immunotargets Ther. 2022;11:11-21. doi:10.2147/ITT.S316602

Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.