- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
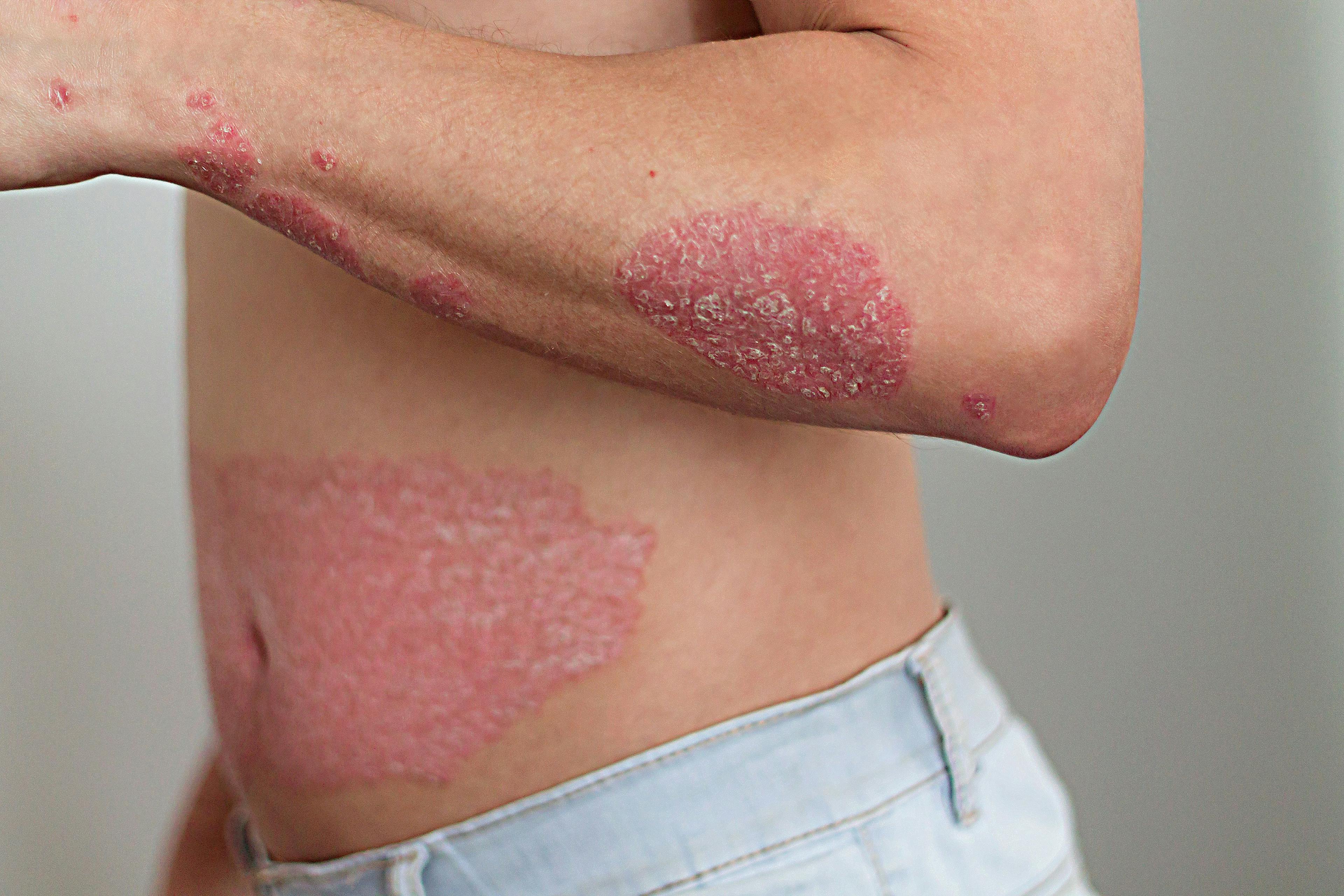
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Publication
Article
Dermatology Times
Innovation Leads to “Windfall” in Dermatology
Author(s):
Continued research and development are resulting in exciting new treatment options for major dermatological conditions.
Exciting developments in the treatment and management of numerous dermatologic diseases are providing much-needed options to adult and pediatric patients with these conditions. New biologics and Janus kinase (JAK) inhibitors offer viable therapeutic choices for an increasing list of dermatologic diseases and conditions, including psoriasis, atopic dermatitis, alopecia areata, vitiligo, and other common dermatoses.
Although the scope of cutaneous conditions and diseases in dermatology is extensive, continued research and development have led to several breakthrough medications to address many common dermatologic diseases.
“In the last 20 years of being involved in dermatology, I have never seen so much innovation and so much of a deep understanding of diseases. I think we have entered the ‘virtuous cycle of drug development,’ where we innovate and make new medicines, learn more about the disease, and then make even better medicines with more targeted actions,” said Peter A. Lio, MD, FAAD, clinical assistant professor of dermatology and pediatrics at Northwestern University Feinberg School of Medicine, founding director of Chicago Integrative Eczema Center, and founding partner of Medical Dermatology Associates of Chicago in Illinois.
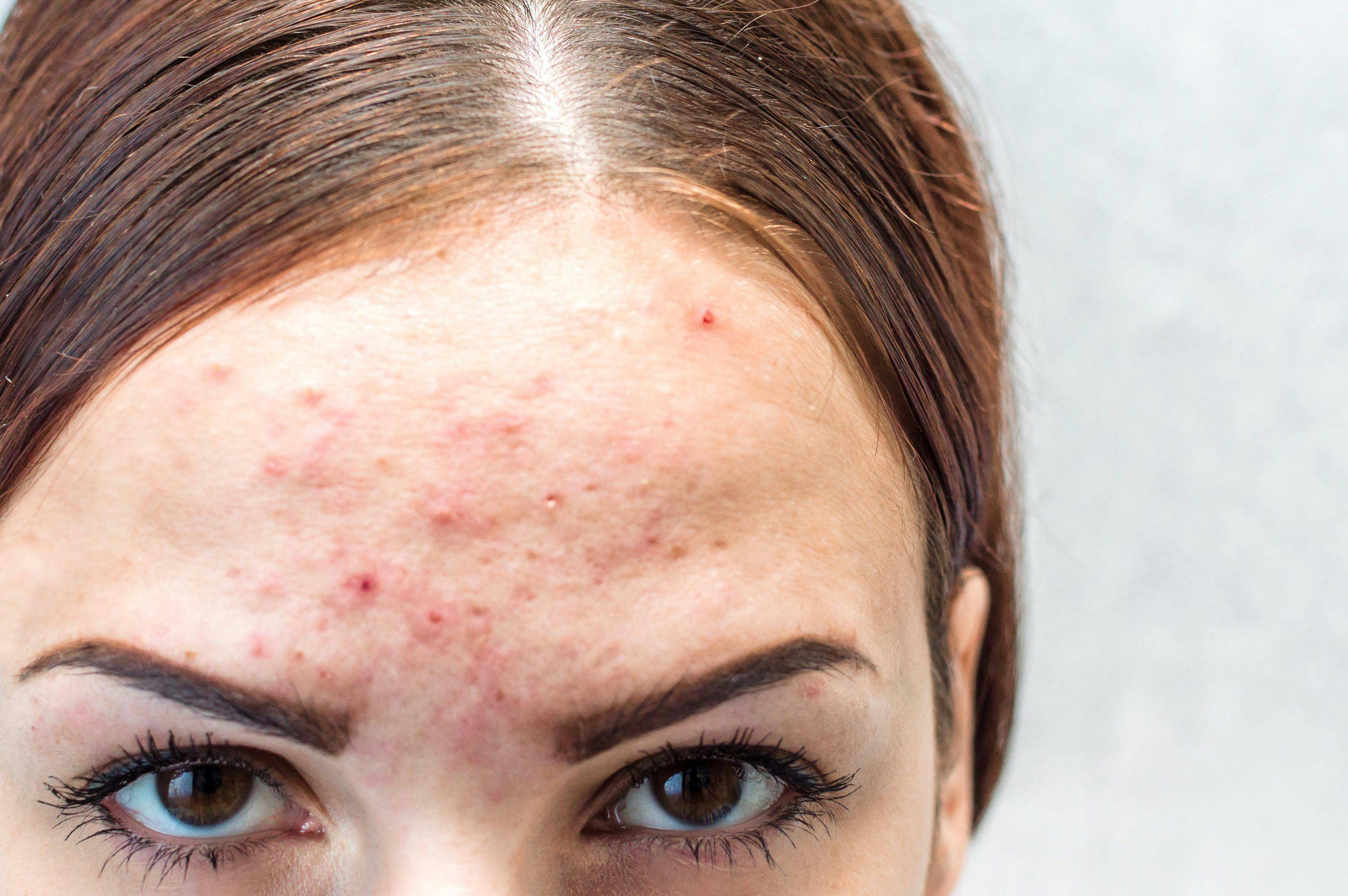
This scenario has played out during the past decade in psoriasis with the availability of ever-improving treatments. According to Lio, this virtuous cycle has continued and expanded to include atopic dermatitis, alopecia areata, vitiligo, and acne.
Recently, new biologic medications that block IL-13 such as tralokinumab-ldrm (Adbry) and pipeline medication lebrikizumab (Lilly) have demonstrated clinical efficacy against moderate to severe atopic dermatitis in adult and adolescent patients. In addition, the FDA approved JAK inhibitors abrocitinib (Cibinqo) and upadacitinib (Rinvoq) in January 2022 for patients with moderate to severe atopic dermatitis and ruxolitinib cream (Opzelura) in September 2021 for mild to moderate cases of the disease. Some of these medications can also be administered to pediatric patients, Lio said, opening the door for a number of breakthrough treatments in this patient population.
“The addition of these excellent therapeutic choices for the entire spectrum of atopic dermatitis has complicated—in a good way—the treatment and management of the disease,” Lio said. “Along with the windfall from all of these new topical and oral medications comes a world of questions in terms of what agent to use and in which scenario. Going forward, we need to discuss the safety of the medications with our patients and carefully determine their optimal treatment plan while understanding the risk factors for each individual patient.”
In dermatology and, in particular, pediatric dermatology, very few treatments are approved for the tremendous range of diseases. As such, many dermatologists have been relegated to live in an off-label world because they know certain medications that lack of FDA approval for an indication or age group could help their patients. According to Lio, the FDA seems to have turned its focus more on children, so pediatric dermatology has benefited recently with the approval of novel medications for younger age groups.
“I am very excited that dupilumab [Dupixent] is now approved down to 6 months of age,” Lio said. “The hope is that we are going to see a number of safe and effective medications that will be made available for a very young pediatric age group, which is hugely important for those physicians who regularly see in the pediatric population.”
Coverage Concerns
Although novel therapeutic agents and drug vehicles for dermatologic diseases have become available on a global scale, insurance providers and health care coverage remain a major obstacle.
“In the last couple of years, we have seen an explosion of new targeted drugs such as dupilumab, which is now the standard of care in most parts of the world,” said Maria Teresa Garcia-Romero, MD, MPH, chair of the International Outreach and Global Alliances Committee for the Society for Pediatric Dermatology and an attending physician and assistant professor at the National Institute of Pediatrics in Mexico City, Mexico. “However, in my region in Latin America, the introduction and approval of this agent as well as other breakthrough medications are still in their beginning stages, and it can be very challenging for patients to receive insurance coverage for these very effective therapies.”
Garcia-Romero said it usually takes 3 to 5 years for these medications, including the IL-17 blocker ixekizumab and IL-12/23 blocker ustekinumab used for psoriasis, to become available in Mexico and other Latin American countries, significantly lagging behind the relatively quicker FDA approval for these medications in the United States. Once approved however, pediatric patients will still have difficulty attaining these agents due to lack of appropriate insurance coverage.
“The accessibility of these drugs is still very difficult, and most patients cannot pay for these medications themselves,” Garcia-Romero said.
“Only those patients who have medical insurance or visit hospitals where government-subsidized plans cover the costs of therapy have access to these drugs. As with other countries, [in Latin America,] unfortunately it is the regulatory environment that hinders patients from [receiving these drugs in a timely manner].”
In addition, many dermatologic diseases and conditions still need to be more urgently addressed, such as alopecia areata, vitiligo, and acne, as well as genodermatoses including congenital ichthyosis and epidermolysis bullosa, the latter of which has almost fully unmet needs. According to Garcia-Romero, some of these diseases for which there isn’t any FDA-approved medication have been experimentally and successfully treated in the past few years with biologics and other targeted therapies. However, repeated attempts to have insurance companies cover the medications for these conditions and diseases have failed, Lio noted, oftentimes leaving patients with the bill or with less optimal treatment options to choose from. Lio added that this can contribute to burnout and exhaustion of clinicians and their patients when seeking optimal therapies.
What’s Next?
“I think some of the next frontiers for novel therapeutic approaches in dermatology are alopecia areata and vitiligo,” Lio said. “Although neither of these diseases have a single FDA-approved treatment to date, I am optimistic that by the end of this year, we will have a working viable treatment for these common dermatologic conditions, likely with the JAK inhibitors leading the way.”
Lio added that as more treatment options become available, it is of paramount importance that the decision-making process regarding treatment and management be shared with the patient. Lio explained that, ideally, clinicians should lay out the potential therapeutic options and carefully review them with the patient when deciding on a treatment plan. Lio said this becomes even more important when dealing with more serious medications because patients must be ready and willing to address any adverse effects that may occur during the treatment period.
“This is a very exciting time in pediatric dermatology and adult dermatology,” Garcia-Romero said. “There are many areas of research in inflammatory disorders and genetic disorders where in the past 10 years, we have seen more advances than in the previous decades. We are getting closer to being able to treat many of the conditions that we see on a daily basis, and we can provide a lasting improvement for patients.”
Lio agreed. “We are currently in an innovation phase of development, and it has been such an exciting bonanza of new ideas and therapies that have recently come forth,” he said. “The next 10 years should be very interesting, and surely our patients will benefit from continuing research in dermatology.”

Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.