- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Publication
Article
Dermatology Times
IL-17 inhibition a path of interest in rosacea treatment
Author(s):
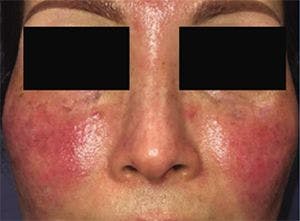
Secukinumab may be safe and effective in patients with moderate-to-severe papulopustular rosacea, a recent study indicates. However, larger, randomized, controlled trials need to confirm benefits.
Interleukin-17 (IL-17) blockade with secukinumab (Cosentyx, Novartis) has recently become a focus of interest as a potential systemic therapy for patients with moderate-to-severe rosacea symptoms.
A recent exploratory study1 looking at IL-17 inhibition with secukinumab found the drug to be safe and effective in patients with moderate-to-severe papulopustular rosacea (PPR).
Rosacea is generally classified into four major subtypes. Milder cases of papulopustular rosacea can be treated with a wide range of topical agents. However, patients with more severe cases of rosacea find topical regimens inadequate. The currently used systemic treatments often fall short of therapeutic goals, begging the need for more effective therapies for this patient population.
“We began studying the biology of rosacea and found, along with other groups, that different pathways are elevated, including IL-17,” says Anne Lynn S. Chang, M.D., professor of dermatology, department of dermatology, Stanford University School of Medicine, Redwood City, Calif., and corresponding author of the study.
As such, IL-17 inhibition through secukinumab seemed like a logical choice, repurposing the drug towards patients with severe rosacea.
Dr. Chang and fellow researchers conducted an exploratory, open-label, single-arm, investigator-initiated clinical trial in 17 adults (six males, 11 females; median age 57 years), evaluating the efficacy and safety of secukinumab for moderate-to-severe PPR.
Study participants received 300 mg of secukinumab weekly for five weeks, and then monthly for two months. Efficacy assessments were performed by three dermatologists blinded to the pre- and post-treatment status images taken during the study. The primary outcome measure was rosacea papule count at week 16 compared to baseline, while secondary outcome measures included Global Erythema Assessment (GEA) score, Global Severity Score (GSS), and self-reported Rosacea Quality of Life Index (RosaQoL) score, at the same follow-up time points.
Among the 17 patients who were available for the final analysis, results showed a significant reduction in papule count at the 16-week follow-up, with a median decrease of -5 papules. Data showed that among patients with the most severe PPR (defined as ≥ 20 inflammatory lesions at baseline), the median papule count was reduced by -22 papules. At the 16-week follow-up, both the GSS and GEA score were significantly reduced, and the median RosaQoL score was improved by -0.6 points.
“I believe that IL-17 inhibitors such as secukinumab could be useful in controlling flares and requires future investment in research to fully understand the potential benefits and risks of this drug class in the severe rosacea population,” Dr. Chang says.
The main side effect experienced by patients was infections, with 39% of patients who received at least one dose experiencing at least one event. However, none were serious, and all patients could be managed by either discontinuation of secukinumab therapy or treatment of their infection.
As rosacea is highly visible, it can be the cause of significant psychosocial distress and anxiety. According to Dr. Chang, treatment with secukinumab appears to address the negative psychosocial impact associated with severe rosacea disease, leading to improvements in quality of life.
“I have patients who are in significant distress because of their rosacea and some of them become desperate due to the severity of their symptoms and the negative impact the disease has on their daily life. We need to increase the treatment options for patients who have failed, cannot tolerate or do not have access to other currently approved treatments,” Dr. Change says.
Although IL-17 inhibition shows activity in moderate-to-severe PPR, Dr. Chang says larger, randomized, placebo-controlled trials still need to be performed to confirm the benefit seen here with secukinumab.
“The space is wide open to explore new and better treatments for severe rosacea and to increase the number of choices that we have based on the science of rosacea information, so that we can eventually better treat our patients,” Dr. Chang says.
Disclosures:
Dr. Chang is an investigator for other studies sponsored by Novartis, Galderma, Regeneron, Merck, and has been an advisory board member for Novartis.
Reference:
1 Kumar AM, Chiou AS, Shih YH, Li S, Chang ALS. An exploratory, open-label, investigator-initiated study of interleukin-17A (IL-17A) blockade in patients with moderate to severe papulopustular rosacea. Br J Dermatol. [Epub ahead of print May 4, 2020]

Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.